Published online Jun 21, 2005. doi: 10.3748/wjg.v11.i23.3518
Revised: May 11, 2004
Accepted: July 12, 2004
Published online: June 21, 2005
AIM: To assess the clinical characteristics of Helicobacter pylori (H pylori) negative duodenal ulcer.
METHODS: Patients with an endoscopic diagnosis of duodenal ulcer between 1996 and 2002 were included in the present study. Patients were considered to be negative for H pylori, if both histological examination and rapid urease test of biopsy specimens were negative. A comparison was made between patients with H pylori positive and negative duodenal ulcers.
RESULTS: A total of 1 343 patients were studied. Their mean age was 54.7±0.5 years. There was a male preponderance (M:F = 2.5:1). Three hundred and ninety-eight patients (29.6%) did not have H pylori infection. The annual proportion of patients with H pylori negative duodenal ulcers increased progressively from 1996 to 2002. On multivariate analysis, patients with H pylori negative duodenal ulcer were more likely to be older, have concomitant medical problem, pre-existing malignancy, recent surgery, underlying sepsis, or taken non-steroidal anti-inflammatory drugs. In terms of clinical presentations, patients with H pylori negative duodenal ulcer were more likely to present with bleeding, multiple ulcers and larger ulcers.
CONCLUSION: The proportion of patients with H pylori negative duodenal ulcers is on the rise because of a continued drop in incidence of H pylori positive duodenal ulcers in recent years. Such patients have distinct clinical characteristics and it is important to ascertain the H pylori status before starting eradication therapy.
-
Citation: Chu KM, Kwok KF, Law S, Wong KH. Patients with
Helicobacter pylori positive and negative duodenal ulcers have distinct clinical characteristics. World J Gastroenterol 2005; 11(23): 3518-3522 - URL: https://www.wjgnet.com/1007-9327/full/v11/i23/3518.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i23.3518
Since the first description of Helicobacter pylori (H pylori), infection with this organism has been considered to be the most important cause of ulcer disease. Previous studies have reported that more than 90% and 60% of patients with duodenal ulcer and gastric ulcer, respectively, were infected with H pylori[1]. The use of non-steroidal anti-inflammatory drugs (NSAIDs) was suggested to be the major cause of the remaining H pylori negative ulcer disease[1,2]. The prevalence of H pylori infection in patients with duodenal ulcer was believed to be so high that confirmatory testing before eradication treatment was considered unnecessary by some centers[3]. It is now apparent that the prevalence of H pylori infection in patients with duodenal ulcer is not as high as what it used to be[4-6].
It is, therefore, important to study the subset of patients with H pylori negative duodenal ulcer in order to assess any other etiologic factors associated with ulcer development and to establish appropriate management strategies. The present prospective study aimed to compare the differences between patients with H pylori positive and negative duodenal ulcer diseases.
Data from all patients with ulcer disease were prospectively fed into a computer database. This database contained data on patients’ demographics, clinical presentations, past history of ulcer disease, concomitant medical problems, pre-existing malignancy, recent history of surgery or sepsis, past surgical history, drug history, family history, social history, vital signs and results of laboratory investigations on admission, amount of blood transfusion given, endoscopic findings (including the location, size and number of lesions), H pylori status, drug treatment prescribed, endoscopic or surgical therapy given, and treatment results. All patients presented to the Department of Surgery, University of Hong Kong Medical Center at Queen Mary Hospital with a diagnosis of ulcer disease were included into the database.
Except for those with active gastrointestinal bleeding, patients were fasted for at least 8 h before endoscopic examination. Endoscopic examination was performed under local pharyngeal anesthesia with the patient lying in a left lateral position. Endoscopic findings were prospectively recorded into the database. An ulcer was defined as a mucosal defect not less than 5 mm in at least one direction. Any mucosal defect of less than 5 mm was classified as erosion. Regardless of the endoscopic findings, three antral mucosal biopsy specimens were taken from within 3 cm of the pylorus and removed from the biopsy forceps (Olympus FB 25K, Olympus, Japan) with a needle. Two biopsy specimens were fixed with 40 g/L formaldehyde for histological examination, while one biopsy specimen was subjected to a rapid urease test[7]. In patients who have taken a proton pump inhibitor, an antibiotic, a bismuth compound, or eradication therapy for H pylori, two additional corpus mucosal biopsy specimens would be sent for histological examination. Patients were considered to be negative for H pylori if both histological examination and rapid urease test were negative. Patients were considered positive for H pylori if any one of the tests was positive.
Patients with an endoscopic diagnosis of duodenal ulcer between 1996 and 2002 were included in the present study. Patients were excluded if (1) they failed to provide an adequate history, including drug history, (2) their H pylori status was not assessed at the time of presentation, (3) they have taken an antibiotic, a bismuth compound, or eradication therapy for H pylori within 3 mo prior to the upper endoscopy, or (3) they refused upper endoscopy. Patients who have taken a proton pump inhibitor were not excluded.
All continuous values were expressed as mean±SE of mean unless otherwise stated. Univariate analysis was performed by Student’s t-test for continuous variables and by χ2 test (with Yates’ correction where appropriate) for categorical variables. Fisher’s exact test was used if any expected cell value in a (2×2) table was less than five. Significant factors identified on univariate analysis were subjected to multivariate stepwise logistic regression analysis. Data analyses were performed with a standard biomedical software package and differences with a P value of less than 0.05 were regarded as statistically significant.
From January 1996 to December 2002, 1343 patients satisfied the inclusion criteria for this study. Their mean age was 54.7±0.5 years. There was a male preponderance (M:F = 2.5:1). Their main presentations included pain (249 patients, 18.5%), bleeding (1074 patients, 80.0%), anemia (14 patients, 1.0%), and obstruction (6 patients, 0.4%).
Three hundred and fourteen patients (23.4%) were smokers, while 61 patients (4.5%) were alcoholics. Four hundred and thirty patients (32.0%) have one or more concomitant medical problems (Table 1). Of these 430 patients, 222 have one, 138 have two, 55 have three, 11 have four, and 4 have five concomitant medical problems. Fifty-five patients (4.1%) gave a past history of pulmonary tuberculosis.
| Concomitant medical problems | Number of patients (%) |
| Hypertension | 279 (20.8) |
| Diabetes mellitus | 127 (9.5) |
| Ischemic heart disease | 79 (5.9) |
| Cerebral vascular accidents | 62 (4.6) |
| Rheumatic diseases | 54 (4.0) |
| Chronic obstructive airway disease | 34 (2.5) |
| Congestive heart failure | 27 (2.0) |
| Chronic renal failure | 26 (1.9) |
| Cirrhosis | 21 (1.6) |
| Asthma | 18 (1.3) |
Sixty-five patients (4.8%) have history of malignancy either currently or in the past, including colorectal cancer (13), urological cancer (10), lung cancer (10), gynecological cancer (8), cancer of nasopharynx (7), breast cancer (5), cancer of larynx (2), liver cancer (2), thyroid cancer (2), tongue cancer (1), lymphoma (1), cancer of maxilla (1), cancer of hypopharynx (1), sarcoma (1), and bone metastasis (1). Thirty-nine patients (2.9%) have undergone surgery within 3 mo before presentation. The natures of the operations are listed in Table 2. Twenty patients (1.5%) have underlying sepsis at the time of presentation, which included chest infection (11), intra-abdominal infection (4), urinary tract infection (3), and orthopedic infection (2). Current medications taken by the patients included NSAIDs (102), aspirin (67), corticosteroids (4), H2 blockers (84), and proton pump inhibitors (15).
| Type of operation | Number of patients |
| Orthopedic | 12 |
| Cardiothoracic | 10 |
| Colorectal | 5 |
| Urology | 4 |
| Neurosurgery | 1 |
| Hepatobiliary | 1 |
| Head and neck | 1 |
| Vascular | 1 |
| Appendicectomy | 1 |
| Gynecology | 1 |
| Endocrine | 1 |
| Ophthalmology | 1 |
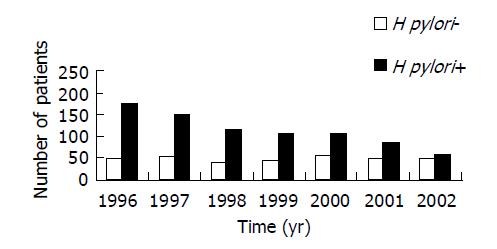
Seventy-six patients (5.7%) have multiple ulcers (more than two ulcers) on presentation. Three hundred and ninety-eight patients (29.6%) did not have H pylori infection. Although the annual proportion of patients with H pylori negative duodenal ulcers increased progressively from 1996 to 2002, the increase was due to an ongoing drop in the number of patients with H pylori positive duodenal ulcers; the annual number of patients with H pylori negative duodenal ulcers has remained relatively constant over the study period (Figure 1).
We analyzed the differences between H pylori positive and negative duodenal ulcers in terms of their pre-existing factors and their clinical presentations.
On univariate analysis, patients with H pylori negative duodenal ulcer were significantly more likely females, older, having concomitant medical problem, having pre-existing malignancy, having recent surgery, having underlying sepsis, taking NSAIDs, taking aspirin, or being non-smokers (Table 3).
| Characteristics | H pylori negative (n = 398) | H pylori positive (n = 945) | P |
| Sex (M/F) | 268/130 | 693/252 | <0.03 |
| Age (yr) | 65.3±0.9 | 50.3±0.6 | <0.0011 |
| Concomitant medical problem (%) | 50.8 | 24.1 | <0.0011 |
| Pre-existing malignancy (%) | 10.3 | 2.5 | <0.0011 |
| Recent surgery (%) | 8.0 | 0.7 | <0.0011 |
| Recent sepsis (%) | 5.0 | 0 | <0.0011 |
| NSAIDs usage (%) | 13.1 | 5.3 | <0.0011 |
| Aspirin usage (%) | 7.3 | 4.0 | 0.019 |
| Corticosteroid usage (%) | 0.8 | 0.1 | 0.08 |
| Smoking (%) | 18.1 | 25.6 | 0.003 |
| Drinking (%) | 3.5 | 5.0 | 0.315 |
On multivariate analysis, six factors were found to be independently associated with H pylori negative duodenal ulcer. These factors included older age, concomitant medical problem, pre-existing malignancy, recent surgery, underlying sepsis, and NSAIDs usage (Table 3).
On analyzing the relationship of each concomitant medical problem with the H pylori status, all except asthma were significantly associated with H pylori negative duodenal ulcer (Table 4).
| Concomitant medical problem (%) | H pylori negative (n = 398) | H pylori positive (n = 945) | P |
| Hypertension | 30.7 | 16.6 | <0.001 |
| Diabetes mellitus | 15.3 | 7.0 | <0.001 |
| Ischemic heart disease | 10.0 | 4.1 | <0.001 |
| Rheumatic diseases | 7.8 | 2.4 | <0.001 |
| Cerebral vascular accidents | 7.3 | 3.5 | <0.005 |
| Congestive heart failure | 5.8 | 0.4 | <0.001 |
| Chronic renal failure | 5.8 | 0.3 | <0.001 |
| Chronic obstructive airway disease | 5.5 | 1.3 | <0.001 |
| Cirrhosis | 4.0 | 0.5 | <0.001 |
| Asthma | 1.0 | 1.5 | NS |
Patients with H pylori negative duodenal ulcer were more likely to present with bleeding, multiple ulcers (i.e., more than two ulcers), and larger ulcers (Table 5).
| Presentations | H pylori negative (n = 398) | H pylori positive (n = 945) | P |
| Presenting symptom | |||
| Presenting symptom Pain | 59 | 190 | |
| Bleeding | 335 | 753 | |
| Obstruction | 4 | 2 | <0.02 |
| Multiple ulcers (%) | 10.6 | 3.6 | <0.001 |
| Ulcer size (cm) | 1.2±0.04 | 0.9±0.02 | <0.001 |
Previous studies have reported that more than 90% of patients with duodenal ulcer are infected with H pylori[1]. The use of NSAIDs was considered as the major cause of H pylori negative ulcer disease[1,2]. Owing to the high prevalence of H pylori infection in patients with duodenal ulcer, patients were given empirical eradication therapy without confirmatory testing of the infection in some centers[3]. Nevertheless, confirmatory testing of H pylori status is considered necessary today in view of a rising prevalence of H pylori negative duodenal ulcer[4-6].
Nowadays it is evident that, apart from H pylori and NSAIDs usage, there remains a group of patients with ulcers of unknown etiology[6]. Besides, there were also arguments put forward to contend against H pylori as the primary cause of duodenal ulcer disease. Firstly, it has not fulfilled all the four Koch’s postulates[8-10]. Secondly, the prevalence of ulcer disease is far lower than the prevalence of H pylori infection[9]. Thirdly, the Indians in Fiji have twice the number of duodenal ulcer than Fijians, although they have similar prevalence of H pylori infection[11,12]. Fourthly, there were numerous recent reports of a rising prevalence of H pylori negative duodenal ulcer[4,5,12,13]. Fifthly, a meta-analysis of North American studies revealed a 20% ulcer recurrence rate within 6 mo of successful eradication of H pylori[14]. The authors concluded that non-H pylori, non-NSAIDS-related ulcer disease may be more common in the USA than previously believed[14].
In view of the significant proportion of H pylori negative duodenal ulcers and the possible existence of causal factors other than H pylori, it is necessary to study the differences between H pylori negative and positive ulcers in order to identify factors other than H pylori, which are involved in the development of ulcer disease.
In the present prospective study of 1 343 patients with endoscopically confirmed duodenal ulcer disease, only 70.4% of patients have H pylori infection. A similar prevalence of H pylori infection in patients with duodenal ulcer was reported by a number of recent studies[5,15]. In view of the current prevalence of H pylori infection in patients with duodenal ulcer, it is important to confirm the H pylori status before prescribing eradication therapy.
It is clear from the present study that the rising proportion of H pylori negative duodenal ulcer was due to a progressive reduction in the number of patients with H pylori positive duodenal ulcer. This is most likely the result of widespread adoption of H pylori eradication for the treatment of H pylori related ulcer both in the primary and in the tertiary care settings in Hong Kong. The different extent in eradication of H pylori in different countries may account for the global variation in the prevalence of H pylori negative duodenal ulcer. The finding that the number of patients with H pylori negative duodenal ulcer has remained static suggested that duodenal ulcer might persist as a health problem unless we are able to identify other etiologic factors which are preventable.
H pylori negative duodenal ulcer was found to be associated with older age, concomitant medical problem, pre-existing malignancy, recent surgery, underlying sepsis, and NSAIDs usage. In the past, the majority of H pylori negative duodenal ulcers was believed to be related to NSAIDs usage[2]. The present study did confirm that NSAIDs is an independent factor associated with H pylori negative duodenal ulcer. However, only about 13% of patients with H pylori negative duodenal ulcer have taken NSAIDs (Table 3). Stress, as a result of recent surgical trauma or underlying sepsis, was also found to be another important risk factor for H pylori negative duodenal ulcer. In fact, it has been known for many years that trauma and sepsis are potent risk factors for the development of ulcer disease[16,17]. Such factors could therefore result in ulcer disease in the absence of H pylori infection.
The association of concomitant medical problem with H pylori negative duodenal ulcer could not be adequately explained. Apart from asthma, all medical problems were significantly associated with H pylori negative duodenal ulcer. Cirrhotic patients were known to have a higher risk of duodenal ulcer independent of H pylori infection[18]. On the other hand, patients with chronic renal failure were known to have a lower rate of H pylori infection[19,20]. The exact reason why concomitant medical problem is related to H pylori negative duodenal ulcer remains to be elucidated.
Older age was also found to be associated with H pylori negative duodenal ulcer in other published studies[13,21]. Physical inactivity was known to be associated with a higher chance of ulcer disease[22,23]. Whether physical inactivity is related to the development of duodenal ulcer in elderly individuals or individuals with underlying medical problem is currently unknown.
It is noteworthy that H pylori negative duodenal ulcer more commonly presented with bleeding, with larger ulcer, or with multiple ulcers. In an elderly patient requiring emergency surgery for bleeding duodenal ulcer, which is usually large in size, acid-reduction surgery may need to be considered in view of the likelihood that the ulcer is unrelated to H pylori infection.
It is unlikely that risk factors identified for H pylori negative duodenal ulcer will vanish in the future. In the presence of an aging population, therefore, it is possible that duodenal ulcer will persist worldwide even with the increasing use of eradication therapy and the availability of more selective COX-2 inhibitors.
This work contributed partly to a chapter of the thesis titled “H pylori infection and gastroduodenal ulcer disease” by Dr. KM Chu for the degree of Master of Surgery at The University of Hong Kong in 2001. The authors would like to thank Dr. Kitty Watt (who was previously an elective medical student from The University of Sydney, Australia), our Research Assistants Ms. Xiao-Mei Zhao, Miss Amy Chan, and Ms. Shing Lui, the nursing and clerical staffs of the Endoscopy Center, and the nursing and clerical staffs of the Upper Gastrointestinal Surgery Ward of the Department of Surgery at Queen Mary Hospital for their contribution and assistance during the study.
| 1. | Kuipers EJ, Thijs JC, Festen HP. The prevalence of Helicobacter pylori in peptic ulcer disease. Aliment Pharmacol Ther. 1995;9 Suppl 2:59-69. [PubMed] |
| 2. | Borody TJ, George LL, Brandl S, Andrews P, Ostapowicz N, Hyland L, Devine M. Helicobacter pylori-negative duodenal ulcer. Am J Gastroenterol. 1991;86:1154-1157. [PubMed] |
| 3. | Massuda HK, Boyd EJ. Who should undergo testing for Helicobacter pylori? Am J Gastroenterol. 1996;91:1070-1071. [PubMed] |
| 4. | Bytzer P, Teglbjaerg PS. Helicobacter pylori-negative duodenal ulcers: prevalence, clinical characteristics, and prognosis--results from a randomized trial with 2-year follow-up. Am J Gastroenterol. 2001;96:1409-1416. [PubMed] |
| 5. | Ciociola AA, McSorley DJ, Turner K, Sykes D, Palmer JB. Helicobacter pylori infection rates in duodenal ulcer patients in the United States may be lower than previously estimated. Am J Gastroenterol. 1999;94:1834-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 137] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Quan C, Talley NJ. Management of peptic ulcer disease not related to Helicobacter pylori or NSAIDs. Am J Gastroenterol. 2002;97:2950-2961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Chu KM, Poon R, Tuen HH, Law SY, Branicki FJ, Wong J. A prospective comparison of locally made rapid urease test and histology for the diagnosis of Helicobacter pylori infection. Gastrointest Endosc. 1997;46:503-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Graham JR. Helicobacter pylori: human pathogen or simply an opportunist? Lancet. 1995;345:1095-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Weiner H, Shapiro AP. Is Helicobacter pylori really the cause of gastroduodenal disease? QJM. 1998;91:707-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Wormsley KG. Campylobacter pylori and ulcer disease--a causal connection? Scand J Gastroenterol Suppl. 1989;160:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Beg F, Oldmeadow M, Morris A, Miller M, Nicholson G. Campylobacter pylori infection in patients undergoing endoscopy in Fiji. N Z Med J. 1988;101:140-141. [PubMed] |
| 12. | Tovey FI, Hobsley M. Is Helicobacter pylori the primary cause of duodenal ulceration? J Gastroenterol Hepatol. 1999;14:1053-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Meucci G, Di Battista R, Abbiati C, Benassi R, Bierti L, Bortoli A, Colombo E, Ferrara A, Prada A, Spinzi G. Prevalence and risk factors of Helicobacter pylori-negative peptic ulcer: a multicenter study. J Clin Gastroenterol. 2000;31:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Laine L, Hopkins RJ, Girardi LS. Has the impact of Helicobacter pylori therapy on ulcer recurrence in the United States been overstated? A meta-analysis of rigorously designed trials. Am J Gastroenterol. 1998;93:1409-1415. [PubMed] |
| 15. | Freston JW. Helicobacter pylori-negative peptic ulcers: frequency and implications for management. J Gastroenterol. 2000;35 Suppl 12:29-32. [PubMed] |
| 16. | Le Gall JR, Mignon FC, Rapin M, Redjemi M, Harari A, Bader JP, Soussy CJ. Acute gastroduodenal lesions related to severe sepsis. Surg Gynecol Obstet. 1976;142:377-380. [PubMed] |
| 17. | Peterson WL. The role of acid in upper gastrointestinal haemorrhage due to ulcer and stress-related mucosal damage. Aliment Pharmacol Ther. 1995;9 Suppl 1:43-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Chen LS, Lin HC, Lee FY, Hou MC, Lee SD. Prevalence of duodenal ulcer in cirrhotic patients and its relation to Helicobacter pylori and portal hypertension. Zhonghua YiXue ZaZhi (Taipei). 1995;56:226-231. [PubMed] |
| 19. | Gladziwa U, Haase G, Handt S, Riehl J, Wietholtz H, Dakshinamurty KV, Glöckner WM, Sieberth HG. Prevalence of Helicobacter pylori in patients with chronic renal failure. Nephrol Dial Transplant. 1993;8:301-306. [PubMed] |
| 20. | Jaspersen D, Fassbinder W, Heinkele P, Kronsbein H, Schorr W, Raschka C, Brennenstuhl M. Significantly lower prevalence of Helicobacter pylori in uremic patients than in patients with normal renal function. J Gastroenterol. 1995;30:585-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Kemppainen H, Räihä I, Kujari H, Sourander L. Characteristics of Helicobacter pylori-negative and -positive peptic ulcer disease. Age Ageing. 1998;27:427-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Cheng Y, Macera CA, Davis DR, Blair SN. Physical activity and peptic ulcers. Does physical activity reduce the risk of developing peptic ulcers? West J Med. 2000;173:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Paffenbarger RS, Wing AL, Hyde RT. Chronic disease in former college students; 13. Early precursors of peptic ulcer. Am J Epidemiol. 1974;100:307-315. [PubMed] |
Science Editor Ma JY and Guo SY Language Editor Elsevier HK