Published online Jun 21, 2005. doi: 10.3748/wjg.v11.i23.3498
Revised: November 18, 2004
Accepted: November 24, 2004
Published online: June 21, 2005
AIM: To investigate the role of overexpression of Bax in apoptotic pathways and the response of human hepatocellular cancer (HCC)-9204 cells to cell death induced by adriamycin.
METHODS: The whole length of Bax cDNA was transfected into human HCC-9204 cells by the method of lipofectamine transfection. An inducible MT-II regulatory system was constructed, which allowed controlled expression of protein upon addition of ZnSO4 (100 μmol/L) as an external inducer. Stable transfecting inducible expression vector containing Bax gene was performed. Expression of Bax in protein was analyzed by immunohistochemistry and Western blotting. TUNEL and flow cytometry were used to assess the effect of Bax on apoptosis. Colony assay and tetrazolium blue (MTT) assay were used to evaluate the difference in drug sensitivity of HCC-9204 cells after Bax-transfection.
RESULTS: Immunohistochemistry and Western blotting demonstrated that the expression of Bax protein markedly increased in Bax-transfected cells 4 h after the addition of ZnSO4. Bax positive signal was frequently found on the cytoplasm and perinuclear region of HCC-9404 cells, and there was ectopic expression in cells with marked condensation of chromatin and cytoplasm (apoptotic cells). Apoptotic index significantly increased in Bax-transfected HCC-9204/Bax cells (3.6 vs 27.2, 4.2 vs 32.3, P<0.05). Flow cytometry analysis showed a significant sub-G1 peak and apoptosis in 15.4% HCC-9204/Bax cells 24 h after treatment. Furthermore, colony survival rate decreased from 66% (HCC-9204/pMD) to 45% (HCC-9204/Bax) 2 d after ADR withdrawal. MTT assay result showed that the effects of Bax on cell viability following ADR exposure were significant as compared to the vehicle-transfected HCC-9204/pMD cells (21% vs 44%, P<0.01).
CONCLUSION: Overexpression of Bax not only induces apoptosis, but also sensitizes HCC-9204 cells to cell death induced by adriamycin.
- Citation: Zheng JY, Yang GS, Wang WZ, Li J, Li KZ, Guan WX, Wang WL. Overexpression of Bax induces apoptosis and enhances drug sensitivity of hepatocellular cancer-9204 cells. World J Gastroenterol 2005; 11(23): 3498-3503
- URL: https://www.wjgnet.com/1007-9327/full/v11/i23/3498.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i23.3498
Apoptosis is a major mode of cell death induced by chemotherapy[1,2]. The efficacy of chemotherapeutic agents depends on their effectiveness in inducing apoptosis of tumor cells[3]. Different tumor cells respond to different chemotherapeutic agents in different ways. Resistance to a variety of cytotoxic chemotherapeutic drugs has become an obstacle in the clinical treatment of human cancers. Bax, as an accelerator of apoptosis, affects response of cancer cells to chemotherapeutic agents and clinical prognosis of cancer patients[4]. Recent studies indicate that overexpression of Bax sensitize human head and gastric cancer cells to various chemotherapeutic agents[5,6], and Bax enhances apoptosis in ovarian cancer cell lines[7].
In the present study, expression of Bax protein was found down-regulated in HCC, and human HCC-9204 cells had a lower Bax gene expression. Thus the HCC-9204 cells can be used as an in vitro model to determine the effect of exogenous Bax on apoptosis and drug sensitivity.
Human hepatocellular carcinoma cell line HCC-9204 was obtained from Department of Pathology, Fourth Military Medical University (Xi’an, China). HCC-9204 cells were cultured in Dulbecco’s modified Eagle’s medium containing phenol red, supplemented with 50 mL/L fetal bovine serum (FBS) in a humidified atmosphere of 950 mL/L air, 50 mL/L CO2 at 37 °C. All the media were supplemented with 2 mmol/L L-glutamine, 0.1 g/L penicillin and 100 kU/L streptomycin. Culture medium and supplements were obtained from Gibco BRL. p53 of the HCC-9204 cell line was mutated.
pBluescript KS-Bax vector containing human full-length cDNA of Bax was presented generously by Dr. Thomas Chittenden (Apoptosis Technology, USA). The MT-II inducible vector pMD-neo was presented by Dr. Jie Zhang ( Institute of Radiation Medicine, China). Complementary DNA of Bax cleaved from the pBluescript KS-Bax plasmid by EcoRI was subcloned into the inducible vector neo-control pMD-neo vector to generate pMD- Bax, including 0.58 kb Bax cDNA and placed under the control of MT-II promoter and the SV40 polyadenylation signal. The inducible vector neo-control pMD-neo and pMD-Bax controlled the expression of protein upon addition of 100 μmol/L zinc as an external inducer. Clonfectin (Clontech, USA) was used to transfect Bax inducible expression vectors into HCC-9204 cells. According to the manufacturer’s instructions, logarithmically growing cells were transfected with 1 μg of plasmids and 2 μL of Clonfectin reagent. For stable transfection, the transfected HCC-9204 cells were selected in medium shown above plus 0.5 g/L G418 (Life Technologies, In USA) for 2 wk, and then the G418 concentration was reduced to 0.2 g/L. Stable cell line of HCC-9204 cells tansfected with pMD-Bax vector was treated by continuous exposure to 100 μmol/L ZnSO4.
HCC-9204 cells were harvested at different time points of induction with ZnSO4. The cell preparations were fixed with 700 mL/L ethanol for 2 h, washed with PBS, and incubated with anti-Bax polyclonal rabbit antibody (Santa Cruz Biotechnology, USA) diluted at 1:100 in PBS containing 10 mL/L bovine serum albumin. Immunostaining was performed using LSAB1 kit (Dako, Peroxidase, USA), according to the manufacturer’s instructions.
Monolayers of the cells were rinsed with PBS and lysed with SDS-PAGE loading buffer (50 mmol/L Tris-HCl pH 6.8, 100 mmol/L dithiothreitol, 2 g/L SDS). Mock and pMD-neo transfected cells served as controls. Samples were analyzed by SDS-PAGE and transferred into Hybond-C super membranes. The membranes were blocked with 50 mL/L skim-milk and 1 g/L Tween-20, then probed with anti-Bax polyclonal rabbit antibody according to the manufacturer’s instructions, washed with PBS and 2 g/L Tween-20 and then incubated with the appropriate horse radish-peroxidase (HRP) conjugated anti-rabbit IgG (Fc). After being washed, the membranes were developed by DAB reagents according to the manufacturer’s guide (Dako Co., USA). The level of β-actin was used as a control for equal loading of protein.
HCC-9024/Bax cells and HCC-9204/pMD cells were harvested for TUNEL staining. The proportion of cells showing DNA fragmentation was measured by incorporation of fluorescein (FITC) -12-dUTP into DNA using terminal deoxynucleotidyltransferase (TdT). The kit was bought from Boehringer Mannheim (in situ cell death detection). Cells were fixed by 40 mL/L paraformaldehyde in PBS overnight at 4 °C. The samples were washed thrice with PBS and permeabilized by 2 mL/L Triton X-100 in PBS for 15 min on ice. After washing twice, cells were equilibrated at room temperature for 15 to 30 min in equilibration buffer with 30 g/L BSA and 200 mL/L normal bovine serum in PBS at pH 7.4, and then the slides were covered with the TUNEL mixture (calf thymus TdT, FITC-12-dUTP and cobalt chloride in 1× reaction buffer ) for 1-2 h at 37 °C in the dark. The tailing reaction was terminated by 2× standard saline citrate (SSC).The samples were washed thrice with PBS and analyzed by fluorescence microscopy. Routine HE staining was also conducted. Negative control was performed by omitting TdT. Quantitative analysis of apoptosis was represented by apoptotic index (AI) as described previously. AI referred to the percentage of at least 1000 counted apoptotic and non-apoptotic cells.
HCC-9204 cells were harvested, including adherent cells (with trypsin-EDTA) and nonadherent cells. Cells were washed with PBS, resuspended and incubated in 700 mL/L ethanol for at least 12 h at 4 °C to permeabilize the plasma membrane. Cells were centrifuged at 1000 r/min and resuspended in 100 mg/L RNase and 10 mg/mL propidium iodide and incubated for 15 min at 25 °C in the dark. Single color fluorescent flow cytometry was performed with a FACS flow cytometer (Becton Dickinson, USA). The histograms were analyzed with Multiplus Software II.
After expression of Bax was induced in HCC-9204 cells through addition of zinc in the culture medium for 24 h, HCC-9204/pMD and HCC-9204/Bax cells were exposed to adriamycin (ADR) at 10 mg/L for at least 4 h, then withdrawn from the culture medium and kept for 24 or 48 h. The following investigations were then conducted. Drug sensitivity was assessed by colony assay and MTT assay.
Soft agar was prepared with 6 g/L bottom agar layer and 4 g/L top agar layer. Culture dishes were fixed and stained with 1 g/L crystal violet at the end of experiment. The number of cells plated for each clone was 2000. Plates were incubated at 37 °C with 50 mL/L CO2 for 7 d. Then the colonies were counted and photographed under a phase contrast microscope (Nikon, Tokyo, Japan). Average colony size and colony cell number were determined by examining several random colonies daily. Cells were tested in triplicate.
HCC-9024/Bax cells and HCC-9204/pMD cells (1×104/L) were seeded in 96-well plates and cultured in 100 μL RPMI medium. After left overnight for adherence, HCC-9024/Bax cells and HCC-9204/pMD cells were divided into two groups. One group was treated with 100 μL ADR, the other group was not treated with any ADR. Cells were treated for different time periods. After treatment, 50 μL of 5 g/L 3-(4,4-dimethylthiazol-2-y1) 2,5-diphenylterazolium bromide (MTT) in PBS was added to each well, incubated for 4 h at 37 °C and the formed formazan crystals were dissolved in 50 μL of dimethyl sulfoxide. The absorbance was recorded at 490 nm on a microplate reader (BioRad). Drug sensitivity was expressed as IC50 for cells.
Data were analyzed by the t test, and P<0.05 was considered statistically significant.
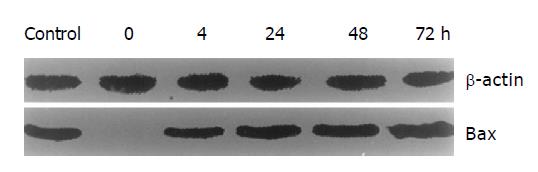
To obtain the regulated Bax expression, pMD- Bax including 0.58 kb Bax cDNA was constructed and transfected into HCC-9204 cells under the control of MT-II promoter. Expression of Bax protein was controlled upon addition of zinc. The stable transfected HCC-9204/Bax cells were selected with G418. Then the cells were exposed to 100 μmol/L ZnSO4. Immunohistochemistry showed that overexpression of Bax was induced by ZnSO4 in HCC-9204/Bax cells (Figure 1). Bax positive signal was frequently found on the cytoplasm and perinuclear region of HCC-9404 cells. Some condensed and round cells existed and demonstrated intense staining in nuclei and cytoplasm of HCC-9204 cells. The Bax ectopic expression in HCC-9204 cells was specific towards cells with marked condensation of chromatin and cytoplasm (apoptotic cells). Furthermore, Western blot analysis demonstrated that Bax was expressed at 0, 4, 24, 48, and 72 h after addition of ZnSO4. Mock and HCC-9204/pMD cells did not express Bax in presence of ZnSO4. Bax expression increase d 4 h after the addition of ZnSO4, and further increased to a steady level at 24 h (Figure 2).
To determine whether overexpression of Bax was able to induce apoptosis in HCC-9204 cells, TUNEL assay was performed. Compared to the pMD-neo transfected HCC-9204/pMD cells, Bax overexpression in HCC-9204/Bax cells showed obvious morphological changes. Some apoptotic cells showed round and condensed cytoplasm and highly fragmented chromatin. TUNEL-positive cells were found with Kelly or yellow fluorescence in nuclei, showing almost no rings. Cells without apoptosis observed by fluorescence microscopy had no fluorescence (Figure 3). Apoptotic index significantly increased in Bax stably-transfected HCC-9204/Bax cells 24 and 48 h after 100 μmol/L ZnSO4 was added (3.6 vs 27.2, 4.2 vs 32.3, P<0.05) .
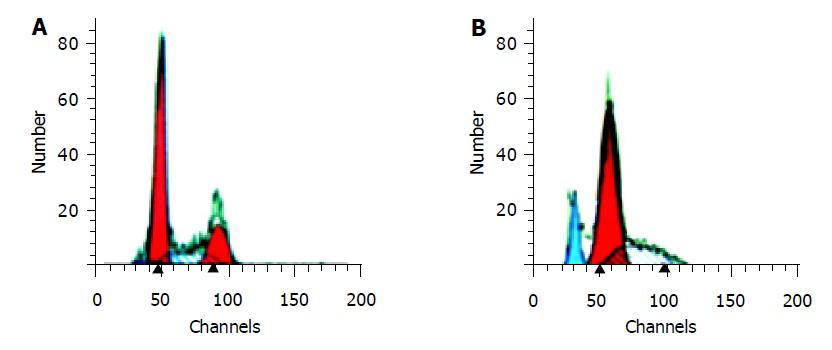
In order to determine the effect of Bax overexpression on apoptosis of HCC-9204 cells, cells were exposed to 100 μmol/L ZnSO4 for 48 h. The apoptotic damage of DNA was detected according to the sub-G1 peak on a flow cytometer. The overexpression of Bax showed a significantly higher apoptotic peak compared to normal control. The apoptotic rate cells was about 15.36% (Figure 4).
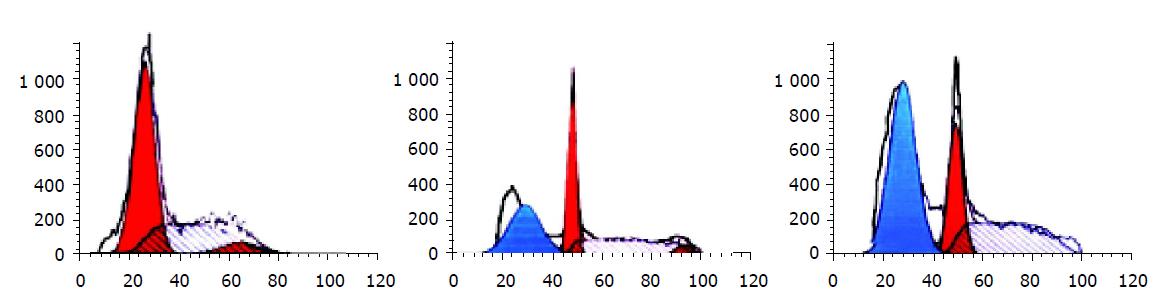
Flow cytometry analysis showed that HCC-9204 cells displayed a significantly higher apoptotic peak than normal control after ADR was added, the apoptotic rate of cells was about 31% and 58% respectively (Figure 5).
The survival of HCC-9204 cells was tested by colony assay following 24 h exposure to adriamycin. The results demonstrated that Bax overexpression caused decrease in colony survival following 24 h exposure to adriamycin. Colony survival rate decreased from 66% (HCC-9204/pMD) to 45% (HCC-9204/Bax ) 2 d after ADR withdrawal (P<0.05 ).
In order to determine the effect of Bax overexpression on drug sensitivity in HCC-9204 cells, MTT assay was performed. Bax-transfected HCC-9204/Bax cells were compared to nontransfected HCC-9204/pMD cells after 48 h exposure to a single optimum concentration of ADR (20 mg/L). MTT assay (Figure 6) showed that the effects of Bax on cell viability following ADR exposure were significant as compared to the vehicle-transfected HCC-9204/pMD cells (P<0.01). The cell viability rate decreased from 44% to 21% 36 h after ADR exposure (P<0.01).
Taken together, overexpression of Bax could not only induce apoptosis, but also sensitize HCC-9204 cells to cell death induced by adriamycin.
Anticancer effects of various chemotherapeutic agents are mediated through a final common pathway: activation of caspase-3 and subsequent DNA fragmentation[8-11]. Proteins of Bcl-2 family are the key regulators of apoptosis[12-14]. Bax, as a proapoptosis gene of the Bcl-2 family, has extensive amino acid homology with Bcl-2, and may form homodimers and heterodimers with Bcl-2 that oppose Bcl-2 function and contribute to cell death[15]. Bax expression is markedly low in esophageal squamous cell carcinoma, breast cancer, hepatocellular carcinoma and ovarian carcinoma[16-19]. Reduced expression of Bax is to be associated with a shorter survival in hepatocellular carcinoma patients[20]. It was reported that Bax-deficient human colon carcinoma cells HCT116 are resistant to cell death[21,22]. Recent studies have shown that overexpression of Bax can modestly induce apoptosis of erythroleukemia cells[4], and sensitize human head and neck squamous cancer cells to various chemotherapeutic agents[5]. Moreover, loss of Bax is associated with the impaired response to therapy[23-25].
Adriamycin (ADR ) is a major antitumor agent for the treatment of a variety of human cancers. Its intracellular effects include free radical formation, inhibition of DNA topoisomerase II, also nucleotide intercalation, resulting in inhibition of DNA replication. As many other chemotherapeutic antitumor drugs, the ensuing induction of apoptosis is likely an important reason for its therapeutic effect. ADR-induced apoptosis typically involves cytochrome C release from mitochondria and subsequent caspase activation[26]. Panaretakis et al[23], found that ADR-induced apoptosis involves the induction of the active conformation of Bak and Bax . But overcoming resistance limits the efficient use of these drugs.
Investigation of the expression and regulation of Bax may provide insights into the mechanisms of susceptibility of HCC to apoptosis induced by chemotherapeutic agents. Our analysis using a gene transfer system demonstrated that overexpression of Bax could not only induce apoptosis, but also sensitize HCC-9204 cells to cell death induced by adriamycin. We presumed that inducible overexpression of Bax might lead to apoptosis. Our study has established that cells overexpressing Bax might be sensitive to ADR during induction of apoptosis. The mechanism might be that Bax initiats cytochrome C release from mitochondria and activates caspase 3 and apoptosis. Overexpression Bax moves to mitochondria and induces cytochrome C release from mitochondria[27]. The release of cytochrome c from mitochondria cleaves and activates caspase-3 and caspase-9, resulting in the sequence of apoptotic processes[28]. Dewson et al[29], found that Bax conformational change and its subcellular redistribution are the common features of B-CLL cell apoptosis in response to diverse stimuli. Kobayashi et al[8], assumed that enhancement of chemotherapeutic agent-induced apoptosis is mediated both by the common pathway of mitochondrial cytochrome C release and by the specific mechanism of each chemotherapeutic agent. Kobayashi et al[4], found that Bax overexpression sensitizes K562 cells to doxorubicin, ara-C and SN-38, but not to etoposide, suggesting that the sensitization effect of Bax is not universal but selective.
Recent reports showed that p53 can induce apoptosis through direct activation of killer genes such as Bax, or down-regulate survival genes such as Bcl-2 in some cell types. p53 is frequently mutated in human cancers, and cells mutated by p53 are more resistant to chemotherapy because of a poorer abiliby to undergo apoptosis[15,30]. Since p53 is mutated in HCC-9204 cells, the downstream molecule Bax may bypass the need for p53 in some forms of chemotherapy-induced apoptosis.
In conclusion, Bax overexpression-based gene therapy in combination with chemotherapy may be an effective treatment modality for HCC.
| 1. | Salah-Eldin AE, Inoue S, Tsukamoto S, Aoi H, Tsuda M. An association of Bcl-2 phosphorylation and Bax localization with their functions after hyperthermia and paclitaxel treatment. Int J Cancer. 2003;103:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Brown JM, Wouters BG. Apoptosis, p53, and tumor cell sensitivity to anticancer agents. Cancer Res. 1999;59:1391-1399. [PubMed] |
| 3. | Domen J, Weissman IL. Hematopoietic stem cells and other hematopoietic cells show broad resistance to chemotherapeutic agents in vivo when overexpressing bcl-2. Exp Hematol. 2003;31:631-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Kobayashi T, Ruan S, Clodi K, Kliche KO, Shiku H, Andreeff M, Zhang W. Overexpression of Bax gene sensitizes K562 erythroleukemia cells to apoptosis induced by selective chemotherapeutic agents. Oncogene. 1998;16:1587-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Guo B, Cao S, Tóth K, Azrak RG, Rustum YM. Overexpression of Bax enhances antitumor activity of chemotherapeutic agents in human head and neck squamous cell carcinoma. Clin Cancer Res. 2000;6:718-724. [PubMed] |
| 6. | Zhao Y, Xiao B, Chen B, Qiao T, Fan D. Upregulation of drug sensitivity of multidrug-resistant SGC7901/VCR human gastric cancer cells by bax gene transduction. Chin Med J (Engl). 2000;113:977-980. [PubMed] |
| 7. | Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590-8607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1107] [Cited by in RCA: 1113] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 8. | Kobayashi T, Sawa H, Morikawa J, Zhang W, Shiku H. Bax induction activates apoptotic cascade via mitochondrial cytochrome c release and Bax overexpression enhances apoptosis induced by chemotherapeutic agents in DLD-1 colon cancer cells. Jpn J Cancer Res. 2000;91:1264-1268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2337] [Cited by in RCA: 2299] [Article Influence: 82.1] [Reference Citation Analysis (1)] |
| 10. | Sakahira H, Enari M, Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391:96-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1194] [Cited by in RCA: 1198] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 11. | Khwaja A, Tatton L. Caspase-mediated proteolysis and activation of protein kinase Cdelta plays a central role in neutrophil apoptosis. Blood. 1999;94:291-301. [PubMed] |
| 12. | Raisova M, Hossini AM, Eberle J, Riebeling C, Wieder T, Sturm I, Daniel PT, Orfanos CE, Geilen CC. The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J Invest Dermatol. 2001;117:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 418] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 13. | Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2873] [Cited by in RCA: 2995] [Article Influence: 124.8] [Reference Citation Analysis (0)] |
| 14. | Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2364] [Cited by in RCA: 2384] [Article Influence: 91.7] [Reference Citation Analysis (0)] |
| 15. | Kirkin V, Joos S, Zörnig M. The role of Bcl-2 family members in tumorigenesis. Biochim Biophys Acta. 2004;1644:229-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 412] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 16. | Beerheide W, Tan YJ, Teng E, Ting AE, Jedpiyawongse A, Srivatanakul P. Downregulation of proapoptotic proteins Bax and Bcl-X(S) in p53 overexpressing hepatocellular carcinomas. Biochem Biophys Res Commun. 2000;273:54-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Thomas A, Pepper C, Hoy T, Bentley P. Bcl-2 and bax expression and chlorambucil-induced apoptosis in the T-cells and leukaemic B-cells of untreated B-cell chronic lymphocytic leukaemia patients. Leuk Res. 2000;24:813-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290:989-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 678] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 19. | Ionov Y, Yamamoto H, Krajewski S, Reed JC, Perucho M. Mutational inactivation of the proapoptotic gene BAX confers selective advantage during tumor clonal evolution. Proc Natl Acad Sci USA. 2000;97:10872-10877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 171] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Garcia EJ, Lawson D, Cotsonis G, Cohen C. Hepatocellular carcinoma and markers of apoptosis (bcl-2, bax, bcl-x): prognostic significance. Appl Immunohistochem Mol Morphol. 2002;10:210-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Ravi R, Bedi A. Requirement of BAX for TRAIL/Apo2L-induced apoptosis of colorectal cancers: synergism with sulindac-mediated inhibition of Bcl-x(L). Cancer Res. 2002;62:1583-1587. [PubMed] |
| 22. | LeBlanc H, Lawrence D, Varfolomeev E, Totpal K, Morlan J, Schow P, Fong S, Schwall R, Sinicropi D, Ashkenazi A. Tumor-cell resistance to death receptor--induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med. 2002;8:274-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 391] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 23. | Panaretakis T, Pokrovskaja K, Shoshan MC, Grandér D. Activation of Bak, Bax, and BH3-only proteins in the apoptotic response to doxorubicin. J Biol Chem. 2002;277:44317-44326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 124] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Eischen CM, Rehg JE, Korsmeyer SJ, Cleveland JL. Loss of Bax alters tumor spectrum and tumor numbers in ARF-deficient mice. Cancer Res. 2002;62:2184-2191. [PubMed] |
| 25. | Eischen CM, Roussel MF, Korsmeyer SJ, Cleveland JL. Bax loss impairs Myc-induced apoptosis and circumvents the selection of p53 mutations during Myc-mediated lymphomagenesis. Mol Cell Biol. 2001;21:7653-7662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 142] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 26. | Gamen S, Anel A, Pérez-Galán P, Lasierra P, Johnson D, Piñeiro A, Naval J. Doxorubicin treatment activates a Z-VAD-sensitive caspase, which causes deltapsim loss, caspase-9 activity, and apoptosis in Jurkat cells. Exp Cell Res. 2000;258:223-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 103] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Jürgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed JC. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci USA. 1998;95:4997-5002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1166] [Cited by in RCA: 1238] [Article Influence: 44.2] [Reference Citation Analysis (12)] |
| 28. | Gao CF, Ren S, Zhang L, Nakajima T, Ichinose S, Hara T, Koike K, Tsuchida N. Caspase-dependent cytosolic release of cytochrome c and membrane translocation of Bax in p53-induced apoptosis. Exp Cell Res. 2001;265:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Dewson G, Snowden RT, Almond JB, Dyer MJ, Cohen GM. Conformational change and mitochondrial translocation of Bax accompany proteasome inhibitor-induced apoptosis of chronic lymphocytic leukemic cells. Oncogene. 2003;22:2643-2654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Lam V, McPherson JP, Salmena L, Lees J, Chu W, Sexsmith E, Hedley DW, Freedman MH, Reed JC, Malkin D. p53 gene status and chemosensitivity of childhood acute lymphoblastic leukemia cells to adriamycin. Leuk Res. 1999;23:871-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |