Published online Jun 14, 2005. doi: 10.3748/wjg.v11.i22.3411
Revised: September 14, 2004
Accepted: December 9, 2004
Published online: June 14, 2005
AIM: To construct and evaluate a polyvalent recombinant vaccine strain Shigella flexneri 2a T32 against enterotoxigenic E.coli (ETEC).
METHODS: By using a host-plasmid balanced lethal system based on asd gene, a polyvalent recombinant strain was constructed to highly express CS3 and regularly express fusion enterotoxin of LTB subunit and mutant ST (LTB/STm) in a vaccine strain Shigella flexneri 2a T32 with specific deletion of asd gene. Fimbria CS3 was observed by immunofluorescence and electron microscopy assay. The security of LTB/STm was examined by ileal loop assay and suckling mouse assay. To evaluate this new candidate vaccine, it was compared with a previous vaccine strain in plasmid and protein level, growth assay and immunogenicity in Balb/c mice.
RESULTS: The newly constructed vaccine expressed CS3 and grew better than the previously constructed vaccine except for the lower expression of LTB/STm. Serum IgG and mucosal IgA against CS3, LTB, ST, and host lipopolysaccharide (LPS) were produced after immunization of Balb/c mice by oral route with the new strain. The titers were not significantly different from the Balb/c mice with the previous strain.
CONCLUSION: This novel candidate diarrheal vaccine can effectively induce serum and mucosal antibody responses against ETEC and Shigella.
-
Citation: Zheng JP, Zhang ZS, Li SQ, Liu XX, Yuan SL, Wang P, Zhan DW, Wang LC, Huang CF. Construction of a novel
Shigella live-vector strain co-expressing CS3 and LTB/STm of enterotoxigenicE.coli . World J Gastroenterol 2005; 11(22): 3411-3418 - URL: https://www.wjgnet.com/1007-9327/full/v11/i22/3411.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i22.3411
Enterotoxigenic E.coli (ETEC) and Shigella are responsible for the high morbidity and mortality caused by diarrheal diseases suffered by infants and children in developing countries, as well as the prevalent causes of diarrhea in travelers from industrialized countries traveling to high risk areas of the world. Infections of these bacteria sometimes are concomitant with some serious complications. Every year more than one million children under the age of five die of diarrhea caused by the pathogens[1,2]. The death rate is much higher under unconventional circumstances such as calamities and wars, which threaten human life and health greatly. It is imperative to develop a safe and effective vaccine against ETEC and Shigella. Colonization factor antigens (CFAs) and enterotoxins are two important factors for ETEC diarrhea, a vaccine containing both types of these antigens together would prevent the ETEC diarrhea effectively. In this research, a main common fimbrial antigen CS3 and two kinds of enterotoxin LT and ST were constructed in FWL01, a Shigella flexneri 2a vaccine T32 without the asd gene. Multi-copy plasmid with strong promoters was used to obtain high expression and significant immune responses.
E.coli strains and plasmids used in this research are listed in Table 1. Shigella strain FWL01, an engineered asd mutant of attenuated strain 2a T32 from wild type strain 2457T, and E. coli X6097 were used as the host strain for Asd+ plasmid[3]. Wild type strain 2457T was used for challenge experiments.
| Strains and plasmids | Characteristics | Reference or source |
| E.coli | ||
| E44815 | CS3, LT, ST | NICPBP |
| X6097 | asd- | Dr. Roy CurissIII |
| S. flexneri | ||
| 2457T | Dr. Wang | |
| FWL01 | (ipa-virg)-, smR, asd-ctxB+ | Dr. Wang[3] |
| Plasmids | ||
| pASD21 | ampR, asd | Dr. Wang[4] |
| pXZL01 | ampR, LTB/STm | This lab[5] |
| pHCSIII | asd, CS3 | This lab[5] |
| pZHY22 | asd, LTB/STm,CS3 | This lab[6] |
| pZCS02 | asd, LTB/STm, CS3 | This study |
Restriction enzymes, bacterial alkaline phosphatase, T4 DNA polymerase, and T4 DNA ligase were products of TaKaRa Co., Ltd (Kyoto, Japan) or England Biolabs (Beverly, MA). Other chemicals were purchased from Sigma (St. Louis, MO).
E.coli strains were routinely incubated in Luria-Bertani (LB) medium supplemented with appropriate antibiotics. The strains carrying CS3 were grown on colonization factor antigen (CFA) agar plates (1% casamino acid, 0.15% yeast extract, 2% agar, 0.04 mmol/L MnCl2, and 0.4 mmol/L MgSO4, pH 7.4 ) at 37 °C for 16 h to express fimbrieal CS3. Toxin medium (20 g/L casamino acids, 10 g/L yeast extract, 5 g/L NaCl, 15 g/L K2HPO4, 2 mg/L MgSO4·7H2O, 2 mg/L FeCl3, 1 mg/L CoCl2·6H2O, 5 g/L glucose, 90 mg/L lincomycin, pH 7.4) was used to incubate wild strain E44815 or recombinant strains for elaboration of enterotoxins.
LTB and polyclonal rabbit anti-serum of LTB were kindly obtained from Meguro-Ku Institute (Tokyo, Japan). Monoclonal antibodies against CS3 and ST were kindly obtained from Ann-Mari Svenneriholm (Sweden).
To prepare recombinant ST fusion protein with glutathione s-transferase (GST), nontoxic mutant ST precursor gene (proximate 200 bp) was cloned into plasmid pGEX-4T-2 of BL21(DE3). Fusion protein was expressed inductively at 37 °C for 2 h and purified with glutathione-linked agarose beads according to the pGEX vector protocol (Phamacia Biotech).
Japanese white rabbits (2-2.5 g), adult guinea pigs, female Balb/c mice at the age of six weeks and three days, respectively were obtained from the Experiment Animal Center of Military Academy of Medical Sciences (Beijing, China). All the animals were maintained in pathogen-free conditions. The mice were randomly divided into two groups of five. Group A was inoculated with the strain FWL01(pZCS02), group B was inoculated with the strain FWL01(pZHY22). The guinea pigs were inoculated with the recombinant strain FWL01(pZCS02) or wild type Shigella flexneri 2a strain 2457T into the conjunctival sac of one eye.
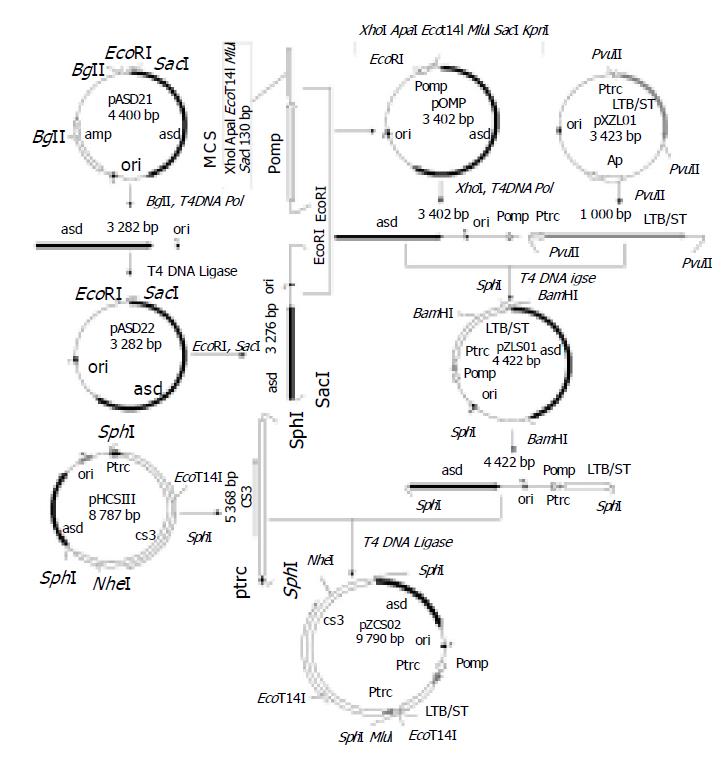
pOMP, the plasmid used for expression of the ETEC antigens in Shigella, was derived from plasmid pUC18 by inserting a asd gene and deleting ampr gene, and then adding a 138bp EcoRI-SacI synthesized promoter PompC containing multiple cloning sites (XhoI, ApaI, EcoT14I, MluI and SacI I) (Figure 1). The ompC promoter was selectively activated by increased osmolarity. To highly express fusion enterotoxin, the fusion gene LTB/STm with promoter trc was cloned as a proximate 1 kbp PvuII- PvuII fragment from plasmid pXZL01 into pOMP downstream of the promoter ompC digested with PvuII, the operon encoding LTB/STm was expressed under promoters trc and ompC. Finally, the 5 368 bp CS3 gene with promoter trc was inserted into this vector from plasmid pHCSIII by SphI digestion. This plasmid co-expressing CS3 and fusion enterotoxin LTB/STm was named pZCS02.
Competent cells of S.flexneri FWL01 were prepared by growing the bacteria in LB broth to an optical density at 600 nm (A600) of 0.4-0.6. The cells were centrifuged, washed twice with cold H2O and once with 10% cold glycerol, and resuspended in the latter at 1/100 of the original volume. A mixture of 50 μL of cells and plasmid DNA was electroporated in 0.2-cm-path cuvette in a gene Pulser (Bio-Rad Laboratories, Hercules, CA) at 2.5 kV, 200 Ω, and 25 uF. Transformants were selected on LB plates containing streptomycin.
The bacteria grown on CFA agar plates at 37 °C for 16 h were harvested and resuspended in PBS. The cells were heated at 60 °C for 30 min and violently vortexed for 1 min[7], then the suspension was sequentially centrifuged at 4 °C, 15000 g for 20 min and at 4 °C, 65000 g for 1.5 h. The precipitate containing CS3 was washed twice in 1% SDS, then dissolved in 0.1 mol/L PB and stored at -20 °C.
The bacteria were grown in CAYE with lincomycin for 16-18 h under conditions of vigorous aeration and agitation. The cells of culture broth were collected and resuspended in 0.1 mol/L PB at 1/20 of the original volume, then lysed by sonication. The crude lysate was clarified by centrifugation and precipitated with sequential ammonium sulfate precipitation (35% and 55% saturation). The precipitate containing LTB/STm was then harvested and dialyzed extensively against 50 mmol/L PB[8], the protein was stored at -20 °C.
Bacterial broth cultures were diluted to a same A600 of 1.0 and one of 1 mL culture was centrifuged and resuspended in 100 μL sample buffer and boiled for 5 min. Aliquots of 5 μL of each sample were electrophoresed on SDS-15% polyacrylamide gels. Gel was transferred to pyroxylin membranes. The membranes were blocked by incubation in PBS-0.05% Tween 20 (PBS-T) containing 5% defatted milk for 2 h, incubated with primary absorbed polyclonal rabbit anti-sera or monoclonal anti-sera antibodies specific for each antigen for 1 h and washed thrice in PBS-T. Then the membranes were incubated in goat anti-rabbit or anti-mouse IgG labeled with horseradish peroxidase specific for primary antibodies at the appropriate concentration for 1 h and washed thrice with PBS-T. The membranes were developed with 3,3’-diaminobenzidine (DAB) and hydrogen peroxide[9].
CS3 fimbria expressed on Shigella was detected by indirect immunofluorescence according to the procedure described previously[10]. The cells were harvested, washed, and resuspended in PBS in a 0.5 mL tube at 1.0 of A600 from CFA plates after 16 h growth, then blocked by PBS-T containing 3% BSA for 30 min, stained with monoclonal antibody of CS3 fimbria diluted to 1:500 for 1 h and with fluorescein isothiocyanate (FITC)-labeled goat anti-mouse immunoglobulins (China) diluted to 1:10 for an additional hour. At last, a drop of stained bacteria was added on glass slides and examined under an Olympus BX50 fluorescence microscope (Olympus Optical Co.) equipped with epi-illumination and interference filters for FITC.
The cells were harvested from CFA plates, resuspended in PBS and placed onto 200-mesh copper grids coated with carbon formvar for 2 min. The grids were then stained for 10 s with 2% phosphotungstic acid (pH 7.2) and examined under a Philips TECHNAI 10 transmission electron microscope at 80 kV[11].
Fresh single colony from LB plate of Shigella containing the recombinant plasmid was inoculated and incubated for 12 h in LB broth at 37 °C and shaken at 200 r/min. A 10-2 dilution of overnight cultures was added to 5.0 mL fresh LB broth with or without 50 μg/mL diaminopimelic acid (DAP) and incubated at 37 °C. After 10 generations (approximately 12 h), the cultures were diluted and the procedure was continued for 100 generations in the same type of media. Then samples of the cultures were diluted to 1/106 into fresh LB broth and spread on LB plates containing DAP. One hundred colonies selected randomly were inoculated on fresh LB plates and recorded the number of new colonies the next day. Ten new colonies were randomly selected for detecting the maintenance of plasmid with CS3 slide agglutination test. Each stability test was performed twice[12].
A single colony of recombinant Shigella was inoculated and incubated in LB medium for 24 h, the A600 of the bacterial culture was recorded every 2 h, then growth curves were drawn.
Security of the recombinant strain was measured by ileal loop assay to detect LT activity. Suckling mouse assay was used to detect ST activity and Sereny keratoconjunctivitis test was performed to detect Shigella activity. The method of ileal loop assay of LT was performed as follows. After being fasted for 48 h, six ileal loops of approximately 10 cm in length were made in an adult Japanese white rabbit. One milliliter of culture filtrates of ETEC-Shigella derivatives was inoculated into each loop as described previously[13]. After 18 h, rabbits were killed and the loops were examined for fluid accumulation. E44815 and FWL01 served as positive and negative control. The result was expressed as milliliters of fluid accumulation per centimeter of loop. Suckling mouse assay of ST was performed as follows. A total of 0.1 mL aliquot of each culture supernatant [FWL01 (pZCS02), E44815, and FWL01] was directly delivered to the stomach of infant mice (three days old) via a flexible plastic tube. Three hours later, each mouse was weighed. The intestine was removed from the mice and weighed, the ratio of gut weight to remaining carcass weight (G/C ratio) was calculated. A G/C ratio of ≥0.083 corresponded to unambiguous accumulation of fluid in the gut lumen and considered as a positive result for ST[14]. To detect the host strain security, recombinant strain was harvested from CFA plates and 10 μL of it was inoculated into the conjunctival sac of one eye of guinea pigs. Inflammatory responses of the inoculated eyes were observed for 5 d. Wild type S. flexneri 2a 2457T served as positive control[15].
Overnight cultures of the immunizing strains were harvested from CFA plates without any antibiotics and suspended in PBS to a concentration of 1010 CFU/mL. Before immunization, the mice were deprived of food and water for 4 h. The mice were incubated with feeding needles for intragastrical delivery of 100 μL of saturated NaHCO3. After 30 min, the mice were administered 200 μL bacterial suspensions and fasted for an additional 30 min. The mice were immunized on d 0, 14, and 28. Blood and fecal samples were collected from mice at pre-immunization and two weeks following each immunization, and serum was obtained by puncturing eyes. The blood was allowed to clot for 30 min at room temperature, centrifuged at 5000 r/min for 10 min, and the serum was collected and stored at -20 °C. Approximately 4 g of feces was added into 1 mL buffer. The fecal pellets were soaked in ice water for 15 min, and vigorously vortexed for 1 min, and the supernatants were stored at -20 °C. To collect intestinal secretions, after the last collection of serum and fecal samples, whole small intestine from the duodenum to the ileocecal junction was excised and broken into pieces, then 3 mL buffer (PBS, 0.01 mol/L EDTA) was added to vortex vigorously for 1 min. After centrifugation at 13000 g for 30 min at 4 °C, supernatants were stored at -20 °C.
Antibodies against CS3, LTB, ST and lipopolysaccharide (LPS) were determined by ELISA[16,17]. Two-fold serially diluted immune serum was added to antigen-coated wells followed by goat anti-serum IgG and IgA. The absorbance was read at 492 nm, Endpoint titers were recorded as the reciprocal of the highest serial dilution of immune serum at which the OD was at least twice that of the non-immune control serum (non-immunized mice) and A492 of the immune serum was at least 0.2. Fecal and intestinal samples were diluted at 1:20 and 1:50. The absorbance at 492 nm represented the antibody response of each sample.
We previously demonstrated the ability of S. flexneri 2a T32 vaccine derivative strain FWL01 to express the ETEC antigens CFA/I, CS3, CS6, LTB, and ST, and to elicit serum IgG and mucosal IgA responses to both ETEC and O antigens of the Shigella vector following intragastric immunization of Balb/c mice[6,18]. The plasmid pZHY22 expressing CS3 and fusion LTB/STm[6] previously constructed was of high molecule (12 kb) which means the low stability and poor growth of strain, although robust immune responses were induced using this recombinant plasmid[6]. To improve the expression system of the plasmid, much lower molecular weight plasmid pZCS02 (9790 bp) was constructed without any antibiotic gene. Promoter trc was engineered into the upstream of CS3 operon and two promoters, ompC, and trc, were cloned into the upstream of fusion enterotoxin gene, in order to enhance the expression of CS3 and fusion enterotoxin. Moreover, the additional multi-cloning site allowed to clone more genes encoding ETEC antigens of interest. This new generation kept the asd gene and colE1 ori for multiple copies in previous plasmid pZHY22. As the asd gene was deleted in the vector strain FWL01, this plasmid-host balanced lethal system by asd has been constructed to minimize plasmid loss from actively growing bacteria without any antibiotic[4-6].
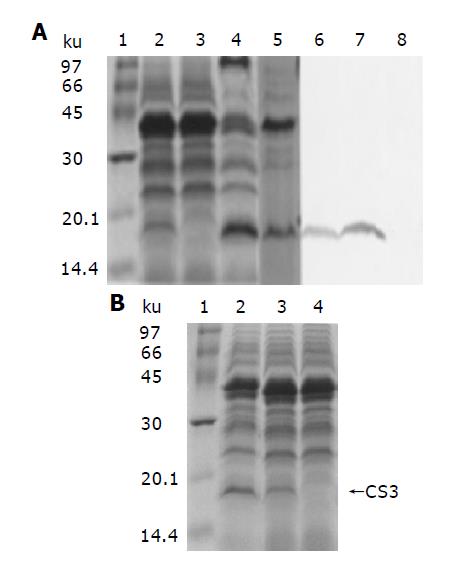
Following electrophoresis with FWL01 (pZCS02) extracts, CS3 fimbria production was detected by Western immunobl-otting assay (Figure 2A). The results indicated that the cloned CS3 operon in pZCS02 of FWL01 encoding a 17.5-ku protein corresponded to the CS3 fimbrial structural subunit. To compare the expression of CS3 in pZCS02 and pZHY22, the whole cell extracts of the two Shigella strains containing one of these plasmids prepared under the same condition were electrophoresed in one gel (Figure 2B). The results showed that the CS3 expression was boosted by promoter trc in plasmid pZCS02.
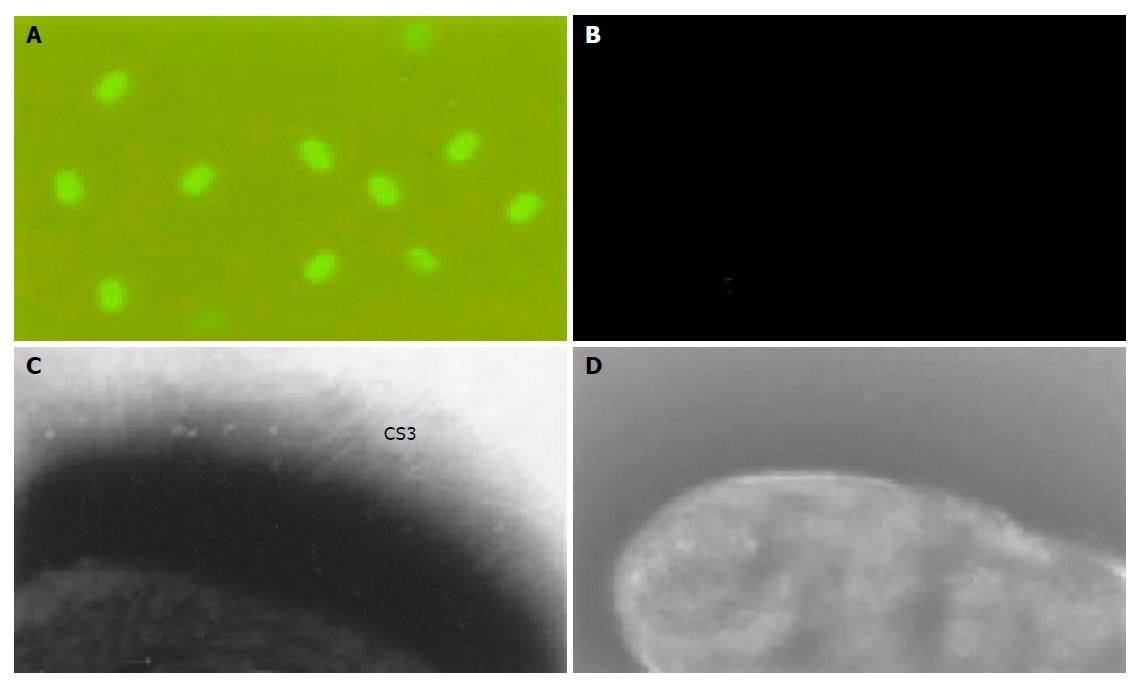
Since ETEC strains could colonize the small-bowel mucosa by means of surface fimbrial antigens and induce antibody responses against these fimbriae to provide protection against ETEC disease, the expression of functional fimbrial CS3 is required for vaccine to inhibit attachment of the ETEC. CS3 expression and typical fimbrial morphology on the surface of vector Shigella strain were detected and confirmed by immunofluorescence and electron microscopy (Figure 3).
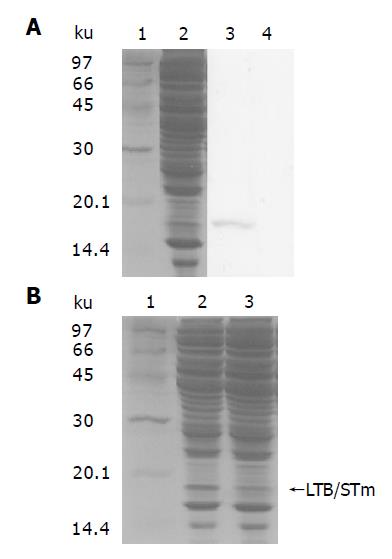
The fusion gene LTB with mutant precursor form of ST at downstream of promoters ompC and trc was designed to expect high expression of this fusion enteroxin by regulation of osmolarity. This mutant ST had two amino acid substitutions at positions 12 and 14 of mature toxin which dramatically diminished the activity (GDP ribosylating) of the toxin and rendered it virtually nontoxic. Therefore the expression of this fusion enterotoxin was driven from the osmotically responsive ompC promoter and trc. Western blot assay indicated the LTB/STm protein was about 18.4 ku (Figure 4A) and had lower expression in plasmid pZCS02 than in vector pZHY22 in Shigella (Figure 4B). The results might be caused by ompC of osmoregulation. The ileal loop and suckling mouse assays (Table 2) exhibited the fusion enterotoxin was safe without LT and ST activities. Sereny keratoconjunctivitis test suggested that all the eyes attacked with FWL01(pZCS02) were not infected, while negative-control animals exhibited full-blown keratoconjunctivitis within three days following conjunctiva challenged with wild type S. flexneri 2a.
| Strain | G/C | Positive(+) Negative(-) | Suckling mice |
| ETEC 44815 | 0.126-0.04 | + | 5 |
| FWL01 | 0.067-0.04 | - | 5 |
| FWL01(pZCS02) | 0.065-0.03 | - | 5 |
To determine whether the module of asd host-plasmid balanced lethal system was efficient, plasmid-containing colonies were grown in LB medium with or without DAP. After 100 generations, all colonies tested in cultures without DAP showed that the plasmid was present in 100% of the cells, whereas only 60% of the cells cultured with DAP contained the plasmid. All the randomly selected colonies were agglutinated with CS3 fimbrial anti-serum. The results suggested that the asd host-plasmid balanced lethal system was an efficient module to stabilize plasmid. The 24 h growth curves showed that the growth of FWL01 (pZCS02) was faster than that of FWL01 (pZHY22) and nearly identical to the carrier FWL01. The results indicated that low molecular pZCS02 and expression of LTB/STm in pZCS02 by osmoregulation reduced the negative influence on FWL01.
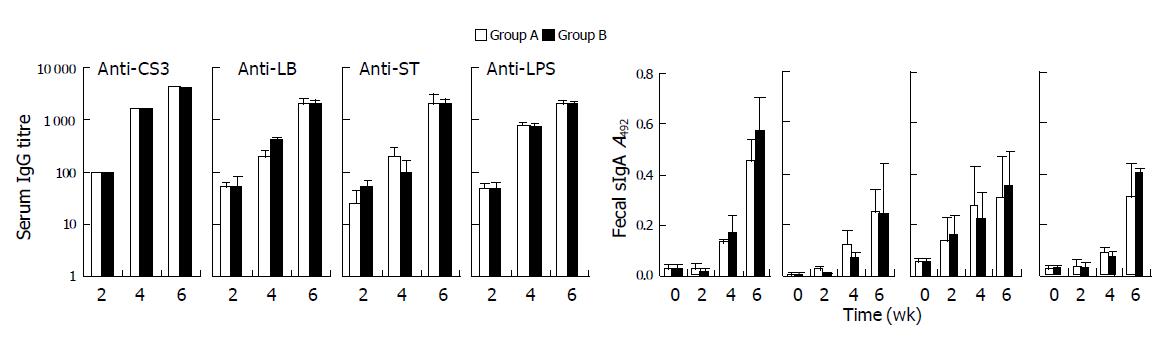
To assess the immunogenicity of the S. flexneri vaccine derivative strain FWL01 containing pZCS02, we studied whether the immune responses to each of the represented antigens from the vaccine were as equivalent as or higher than those elicited by the previously constructed strain FWL01(pZHY22). Balb/c mice were immunized with the strains FWL01(pZCS02) and FWL01(pZHY22), respectively. The results of the immunogenicity study are summarized in Figure 5 and Table 3. Both groups responded to ETEC antigens and Shigella vector itself with both serum IgG and mucosal antibodies against CS3, LTB, ST, and LPS. Each response observed following the primary immunization was boosted to higher levels following the second immunization. Moreover, the two groups elicited approximately equivalent antibody responses following each immunization. However, the mucosal responses to CS3 of group A immunized with FWL01 (pZCS02) were somewhat lower than those of group B immunized with FWL01 (pZHY22), though the difference was less than two-fold and not statistically significant.
| Intestinal sIgA | Anti-CS3 | Anti-LTB | Anti-ST | Anti-LPS |
| Negative control | 0.109±0.001 | 0.144±0.004 | 0.077±0.003 | 0.121±0.005 |
| FWL01(pZHY22) | 0.972±0.191 | 0.570±0.270 | 0.172±0.023 | 0.558±0.121 |
| FWL01(pZCS02) | 0.741±0.153 | 0.803±0.371 | 0.259±0.088 | 0.384±0.082 |
In human ETEC diarrheal infection, fimbria-mediated attachment to enterocytes of the proximal small intestine and enterotoxin production are involved in the pathogenesis and offer targets for immunoprophylaxis. Approximately 35% of enterotoxins express LT and ST, and about 35% express only ST and the rest express only LT of the ETEC strain[19]. Twenty different colonization factor antigens of CFAs in human ETEC have been described[19]. Epidemiological studies carried out in many geographical areas have shown that the occurrence of different CFAs of human ETEC varies[20]. But the most prevalent antigenic CFA/I and the complex of CS1-CS6 have been found in 50-80% of clinical human ETEC isolates[21]. These seven fimbrial types are considered essential in future ETEC vaccine to elicit broad-spectrum protection. CFAs have been shown to cooperate synergistically with ETEC toxin antigen in providing protection against ETEC[22]. Effective ETEC vaccine should be given orally and evoke both anticolonization and antitoxic immune responses in the intestine.
Shigellae cause diseases by invading the colonic epithelium through specialized M cells and then spread from cell to cell with inflammatory responses, leading to cell death and dysentery[23]. There are four species of Shigellae. S. dysenteriae (group A), S. flexneri (group B), S. boydii (group C) and S. sonner (group D). S. flexneri serotypes are the most common agents of endemic shigellosis in developing countries[24]. Protective immunity against Shigella is serogroup and largely serotype-specific, as it is targeted against epitopes residing within the O polysaccharide of LPS. Live, oral, attenuated vaccine strains are safe and effective in eliciting protective immunity.
Attenuated Shigella vaccine strain exhibits as a live vector for ETEC antigens within the gut-associated lymphoid tissue. In order to create a multivalent vaccine against both Shigella and ETEC, we used live attenuated strains of S. flexneri 2a as a vector for expression of CFA/I, CS6, CS3, and fusion enterotoxin LTB/STm. These live vector constructs are able to elicit both serum and mucosal immune responses against O antigens of the Shigella vector and the ETEC antigens following intragastric immunization in the Balb/c mice model. We have previously engineered both CS3 and nontoxic fusion enterotoxin LTB/STm with promoter trc into derivative plasmid pBR322 to construct pZHY22 in FWL01. The low level of CS3 expression and poor growth of the ETEC-Shigella strain were up to our expectation, though each ETEC antigen had high immunogenicity[6].
In this study we constructed pZCS02, a novel plasmid carrying the genetic determinants encoding CS3 and fusion LTB/STm derivate from pUC18. There is evidence that the expression level of CS3 is higher in pUC18 than in pBR322[18]. Based on the previous plasmid pZHY22, we cloned a trc promoter into the upstream of CS3 and an ompC promoter into the upstream of trc promoter and fusion enterotoxin LTB/STm into pZCS02. Thus this plasmid (9790 bp) is smaller than pZHY22 (12000 bp). In theory, Promoter can enhance expression of the downstream gene. The results showed that the expression of CS3 in pZCS02 was higher than that in pZHY22. Moreover, the growth of Shigella containing pZCS02 was much better than that of Shigella containing pZHY22. These advantages are required for ETEC vaccine. But the expression of LTB/STm was unexpectedly lower than that of pZHY22, which may be caused by the ompC activity of osmoregulation.
It was also demonstrated in our work that immunization with FWL01 (pZCS02) elicited immune responses not only to CS3, LTB and ST, but also Shigella O antigen. Specific serum and mucosal antibody responses were generated against ETEC antigens. Moreover, these responses were approximately similar to those elicited by immunization with live vector FWL01 carrying pZHY22. The immune responses against CS3 even decreased slightly. One reasonable explanation is the lower expression of LTB/STm. LTB like CTB, is a potent adjuvant, which influences the immune response against CS3.
In theory, surface expression of ETEC fimbrial antigens and elaboration of ETEC enterotoxins can alter the immunogenicity and safety of the shigella vector. However, the two groups of Balb/c mice immunized with this Shigella live vector expressing both ETEC fimbrial and enterotoxic antigens had high serum and mucosal responses. Guinea pigs challenged with this vaccine containing ETEC antigens had no inflammatory response compared to 100% keratoconjunctivis after inoculation of wild type shigella.
It should be noted that the asd system not only stably maintains the asd+ plasmid in asd mutant vector FWL01, but also eliminates the need of drug resistance markers, thus avoiding use of antibiotics during fermentation of vaccine. FWL01 is a derivate of S. flexneri 2a vaccine T32 without asd. Every year, 62.8-77.3% of shigellosis are caused by S. flexneri in China[25]. We have engineered CFA/I and CS6 in FWL01 and obtained satisfactory immune responses to the two antigens in a Balb/c mice model[26]. This novel vector vaccine may play an important role in preventing diarrheal diseases caused by ETEC and S. flexneri 2a.
We thank Professor Yang Xiao and Ms. Yu-Chuan Li for immunofluorescence and electron microscopy facilities.
| 1. | Gilligan PH. Escherichia coli. EAEC, EHEC, EIEC, ETEC. Clin Lab Med. 1999;19:505-521. [PubMed] |
| 2. | Lindberg AA, Pál T. Strategies for development of potential candidate Shigella vaccines. Vaccine. 1993;11:168-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Wang HL, Feng EL, Lin Y, Liao X, Su GF. Construction of △asd mutant of Shigella flexneri 2a strain T32. Junshi Yixue Kexueyuan Yuankan. 2000;24:81-87. |
| 4. | Wang HL, Feng EL, Lin Y, Su GF. Coloning and sequencing of shigella flexneri 2a asd gene. Weishengwuxue Yu Mianyixue Jinzhan. 2000;28:15-19. |
| 5. | Xu B, Zhang ZS, Li SQ, Shu D, Yu SY, Huang CF. Construction of the attenuated Salmonella typhimurium strain expressing Escherichia coli LT-B/ST fusion antigens. Junshi Yixue Kexueyuan Yuankan. 1999;23:172-174. |
| 6. | Liu TT, Li SQ, Zhang ZS, Zheng JP, Liu XL, Luo G, Huang CF. Simultaneous expression of CS3 colonization factor antigen and LT-B/ST fusion enterotoxin antigen of enterotoxigenic Escherichia coli by attenuated shigella flexneri 2a. Shengwu Huaxue Yu Shengwu Wuli Xuebao. 2003;35:49-54. |
| 7. | Levine MM, Ristaino P, Marley G, Smyth C, Knutton S, Boedeker E, Black R, Young C, Clements ML, Cheney C. Coli surface antigens 1 and 3 of colonization factor antigen II-positive enterotoxigenic Escherichia coli: morphology, purification, and immune responses in humans. Infect Immun. 1984;44:409-420. [PubMed] |
| 8. | Zhang ZS, Li SQ, Dong ZZ, Huang CF. Fusion of genes enterotoxigenic Escherichia coli heat-liable and heat-stable enterotoxins. Zhonghua Weishengwuxue He Mianyixue Zazhi. 1994;14:219-222. |
| 9. | Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual, 2nd ed. New York: Cold Spring Harbor Laboratory Press 1989; 880-898. |
| 10. | Francisco JA, Campbell R, Iverson BL, Georgiou G. Production and fluorescence-activated cell sorting of Escherichia coli expressing a functional antibody fragment on the external surface. Proc Natl Acad Sci USA. 1993;90:10444-10448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 164] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Meacock PA, Cohen SN. Partitioning of bacterial plasmids during cell division: a cis-acting locus that accomplishes stable plasmid inheritance. Cell. 1980;20:529-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 269] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Nakayama K, Kelly SM, Curtiss R 3rd. Construction of an ASD expression-cloning vector: stable maintenance and high level expression of cloned genes in a salmonella vaccine strain. Biol Technol. 1988;6:693-697. [RCA] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 170] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Aitken R, Hirst TR. Recombinant enterotoxins as vaccines against Escherichia coli-mediated diarrhoea. Vaccine. 1993;11:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Giannella RA. Suckling mouse model for detection of heat-stable Escherichia coli enterotoxin: characteristics of the model. Infect Immun. 1976;14:95-99. [PubMed] |
| 15. | Sereny B. Experimental keratoconjunctivitis shigellosa. Acta Microbiol Acad Sci Hung. 1957;4:367-376. |
| 16. | Li SQ, Zhang ZS, Chen TM, Huang CF. Detection of colonization factor antigen I of enterotoxigenic Escherichia coli through ELISA. Jiefangjun Yixue Zazhi. 1988;13:271-273. |
| 17. | Ahrén C, Wennerås C, Holmgren J, Svennerholm AM. Intestinal antibody response after oral immunization with a prototype cholera B subunit-colonization factor antigen enterotoxigenic Escherichia coli vaccine. Vaccine. 1993;11:929-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Dong ZZ, Zhang ZS, LI SQ, Zhang BN, Huang CF. Clonging and expression of the gene encoding CS3 fimbriae antigen of enterotoxigenic Escherichia coli. Zhonghua Weishengwuxue He Mianyixue Zazhi. 1994;14:84-88. |
| 19. | Gaastra W, Svennerholm AM. Colonization factors of human enterotoxigenic Escherichia coli (ETEC). Trends Microbiol. 1996;4:444-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 339] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 20. | Sommerfelt H, Steinsland H, Grewal HM, Viboud GI, Bhandari N, Gaastra W, Svennerholm AM, Bhan MK. Colonization factors of enterotoxigenic Escherichia coli isolated from children in north India. J Infect Dis. 1996;174:768-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Girón JA, Xu JG, González CR, Hone D, Kaper JB, Levine MM. Simultaneous expression of CFA/I and CS3 colonization factor antigens of enterotoxigenic Escherichia coli by delta aroC, delta aroD Salmonella typhi vaccine strain CVD 908. Vaccine. 1995;13:939-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Kaper JB, Levine MM. Progress towards a vaccine against enterotoxigenic Escherichia coli. Vaccine. 1988;6:197-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Sansonetti PJ. Microbes and microbial toxins: paradigms for microbial-mucosal interactions III. Shigellosis: from symptoms to molecular pathogenesis. Am J Physiol Gastrointest Liver Physiol. 2001;280:G319-G323. [PubMed] |
| 24. | Kotloff KL, Winickoff JP, Ivanoff B, Clemens JD, Swerdlow DL, Sansonetti PJ, Adak GK, Levine MM. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ. 1999;77:651-666. [PubMed] |
| 25. | Pan SW. Epidemic Shiga bacillus dysentery in China. Zhonghua LiuXingBingXue ZaZhi. 1988;9:59-62. [PubMed] |
| 26. | Zheng JP, Wang LC, Luo G, Li SQ, Duan HQ, Huang CF, Zhang ZS. Co-expression of CFA/I and CS6 of enterotoxingenic Escherichia coli (ETEC) in Shigella flexneri 2a T32 derivative strain FWL01. Shengwu Huaxue Yu Shengwu Wuli Xuebao. 2003;35:1005-1010. |