Published online Jun 14, 2005. doi: 10.3748/wjg.v11.i22.3392
Revised: July 1, 2004
Accepted: August 2, 2004
Published online: June 14, 2005
AIM: To establish and characterize a new cholangiocarcinoma cell line from a patient living in the Opisthorchis viverrini (O. viverrini) endemic area of Northeast Thailand.
METHODS: Fresh liver biopsy and bile specimens were obtained from a 65-year-old Thai woman with cholangiocarcinoma of the porta hepatis. After digestion, the cells were cultured in Ham’s F12 media. The established cell line was then characterized for growth kinetics, cell morphology, imm-unocytochemistry and cytogenetics. Tumorigenicity of the cell line was determined by heterotransplanting in nude mice.
RESULTS: The primary tumor was a poorly differentiated tubular adenocarcinoma. Examination of the bile revealed malignant cells with O. viverrini eggs. The cholangioc-arcinoma cell line KKU-100 was established 4 mo after the primary culture-population doubling time was 72 h. KKU-100 possesses compact and polygonal-shaped epithelial cells. Immunocytochemically, this cell line exhibited cytokeratin, EMA, CEA, and CA125, but not α-fetoprotein (AFP), CA19-9, desmin, c-met, or p53. Such protein expressions parallel those of the primary tumor. Cytogenetic analysis identified aneuploidy karyotypes with a modal chromosome number of 78 and marked chromosomal structural changes. Inoculation of KKU-100 cells into nude mice produced a transplantable, poorly differentiated aden-ocarcinoma, similar to the original tumor.
CONCLUSION: KKU-100 is the first egg-proven, Opisthorchis-associated cholangiocarcinoma cell line, which should prove useful for further investigations of the tumor biology of this cancer.
- Citation: Sripa B, Leungwattanawanit S, Nitta T, Wongkham C, Bhudhisawasdi V, Puapairoj A, Sripa C, Miwa M. Establishment and characterization of an opisthorchiasis-associated cholangiocarcinoma cell line (KKU-100). World J Gastroenterol 2005; 11(22): 3392-3397
- URL: https://www.wjgnet.com/1007-9327/full/v11/i22/3392.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i22.3392
Cholangiocarcinoma is rare but its prevalence in Northeast Thailand makes the region the area of highest incidence in the world[1]. Coincidentally, Thailand has a high prevalence of infection of the liver fluke, Opisthorchis viverrini, and an estimated six million people are infected[2]. Both experimental and epidemiological evidence implicate opisthorchiasis in the etiology of cholangiocarcinoma in this endemic area[3,4]. Cumulative data suggest that the pathogenesis of this bile duct cancer in this region is different from that observed in Western countries with different etiologies[4]. However, the detailed mechanisms of the cellular and molecular pathogenesis of Opisthorchis-associated cholangiocarcinoma and its biology are unclear.
Human solid tumor cell lines are important sources for studies of tumor biology including tumor cell growth, differentiation, metastasis, molecular pathogenesis, and susceptibility to drugs. A small number of reports, compared to other cancers, describing cholangiocarcinoma cell lines have been published, most from extrahepatic cholangiocarcinoma[5-9] and a few from intrahepatic cholangiocarcinoma[9,10-13]. Only one cholangiocarcinoma cell line was developed from a Thai[14]. We therefore aimed to establish more cholangiocarcinoma cell lines from patients living in Northeast Thailand. Our report describes the establishment and characterization of an egg-proven, Opisthorchis-associated cholangiocarcinoma cell line, developed in our laboratory and designated as KKU-100.
A 65-year old Thai woman was admitted to the Faculty of Medicine, Srinagarind Hospital, Khon Kaen University, Thailand. She arrived with nausea, progressive jaundice and itching. She was previously diagnosed by ultrasonography at a provincial hospital as having cholangiocarcinoma. Significant laboratory investigations at admission showed elevated alkaline phosphatase (ALP) (266 U/mL, normal 42-120 U/mL), total bilirubin (23.1 mg/mL, normal 0.25-1.5 mg/mL), carcinoembryonic antigens (CEA) (44.6 ng/mL, normal <2.5 ng/mL), while the serum α-fetoprotein (AFP) (2.8 U/mL, normal <10 U/mL), HBsAg and HBsAg were negative. The stool examination revealed O. viverrini eggs. The serum counterpart was positive for the parasite-specific antibody using ELISA.
The diagnosis of cholangiocarcinoma was confirmed by ultrasonography, computer tomography, and endoscopic retrograde cholangiopancreatography (ERCP). Operative findings revealed skipped tumor lesions at the porta hepatis with bile duct obstruction and ascites (approximately 1000 mL). The cul-de-sac, peritoneum and bowel wall were tumor free. A bilateral peripheral hepatojejunostomy (Roux-en-Y) with cystojejunostomy was the palliative treatment performed. Liver biopsy of the tumor mass was taken, the histopathology indicated a poorly differentiated tubular adenocarcinoma. Biliary cytology revealed clusters of tumor cells with O. viverrini eggs. The patient developed sepsis and died two follow-ups after surgery. Informed consent was obtained from the patient.
The liver biopsy was transported to the Department of Pathology immediately after surgery and used for cell culture. The tumor tissue was aseptically washed in Ham’s F12 media (Seromed, Berlin, Germany) containing penicillin (200 U/mL) and streptomycin (200 μg/mL), minced, then digested with 0.25% trypsin-EDTA (Gibco/BRL, Grand Island, MA) for 1 h at 37 °C. After dissociation, the tissue fragments were force-filtered through a 100-μm pore mesh and the cell suspension was washed in the media 2-3 times to remove any residual blood. The washed cells were then cultured in Ham’s F12 containing penicillin (100 U/mL) and streptomycin (100 μg/mL) with 20% fetal bovine serum (Gibco/BRL) at 37 °C with 50 mL/L CO2, the media were changed twice, weekly. Cultured tumor cells were observed daily under a phase-contrast microscope (Olympus, Tokyo, Japan). Contaminating fibroblasts were periodically removed with a cell scraper (Costar, Cambridge, MA) and by differential trypsinization.
After establishment, growth kinetics of the tumor cells was obtained by seeding the cells at 2×105 cells/well in duplicate in a 24-well plate (Nunc, Roskilde, Denmark). After seeding, the media were changed every two days. The cells were detached from the wells with trypsin-EDTA and the average number of viable cells was counted every 24 h in a hemacyt-ochamber in the presence of trypan-blue dye. Cells were counted for up to 12 d. The doubling time of the cell population was estimated during the logarithmic growth phase.
Tissue culture flasks were routinely monitored for cell morphology under a phase-contrast microscope.
Four- to six-week-old athymic nude mice (BALB/cAnNCrj-nu/nu) were used for heterotransplantation. The mice were injected subcutaneously with KKU-100 cells (1×107 cells) suspended in culture medium and kept under specific pathogen-free conditions. The tumor was observed every week and when it reached a diameter of ≥1.5 cm, the mice were killed. The tumor was minced and transplanted again to other nude mice for serial transplantation. A portion of tumor was fixed in 10% buffered formalin, routinely processed for histopathology and immunocytochemistry.
To characterize the expression of cytokeratin, tumor markers and some gene products, the cells were grown as a confluent monolayer, washed in cold PBS, then scraped. After centrif-ugation, the cell pellet was fixed in buffered formalin and routinely processed in a cell block. Paraffin sections were cut and immunostained for cytokeratin, CEA, AFP, CA125, CA19-9, c-met and p53 using the standard avidin-biotin peroxidase method[15]. Details of the antibodies are shown in Table 1. Mouse monoclonal antibody to pancytokeratins (Dako, Denmark, clone MNF 116) reacts with cytokeratins 5, 6, 8, 17, and probably 19 as shown in the specification sheet of the manufacturer. Primary and heterotransplanted tumors were similarly immunostained.
| Antibodies | Sources | Primary tumor1 | KKU-100 cells1 | Heterotrans-planted tumor1 |
| Cytokeratin (clone MNF116) | Dako, Denmark | +++ | ++ | - |
| EMA (clone E29) | Dako, Denmark | ++ | ++ | +++ |
| Desmin (clone D33) | Dako, Denmark | - | - | - |
| CEA (polyclonal) | Dako, Denmark | +++ | + | - |
| AFP (polyclonal) | Dako, Denmark | |||
| CA19-9 (clone 116-NS-19-9) | Dako, Denmark | - | - | - |
| CA125 (clone Ov185:1) | Novocastra, UK | +++ | + | - |
| c-Met (polyclonal) | Santa Cruz, USA | - | - | - |
| p53 (clone DO-7) | Dako, Denmark | - | - | - |
After 20 passages, the established tumor cells at the exponential phase were subjected to chromosomal analysis by treating them with 0.1 μg/mL colcemid (Gibco/BRL). The cells were treated with a KCl/HEPES/EDTA hypotonic solution and harvested according to standard cytogenetic procedures. Slides of fixed cells were trypsin-Giemsa-banded to identify individual metaphase chromosomes. Representative chromosome sets were photographed and karyotyped. The modal chromosome number was determined from 100 cells.
Direct agar incubation of spent medium using a mycoplasma agar plate (Gibco/BRL) was routinely performed to detect any mycoplasma contamination.
A few days after the primary culture, a few single colonies of epithelial-like round cells and fibroblasts attached to the 25 mL culture flask. The colonies were inactive and stable for several weeks while fibroblasts grew faster. Fibroblasts were periodically removed by scraping. Three months after the cell culture was initiated, the epithelial-like cells became active and propagated as monolayer cells. The colonies were then subcultured with trypsin-EDTA. Contaminating fibroblasts were removed by scraping and differential trypsinization. After four successive passages, a pure tumor cell line with continuous, stabilized multiplication was established and designated as KKU-100. The cell line KKU-100 was maintained in our laboratory in >100 passages over the past 5 years.
KKU-100 cells followed a typical growth curve, including lag, logarithmic and stationary phases during the 12-d culture. By the tenth passage, the population doubling time in Ham’s F12 with 10% fetal bovine serum, was approximately 72 h.
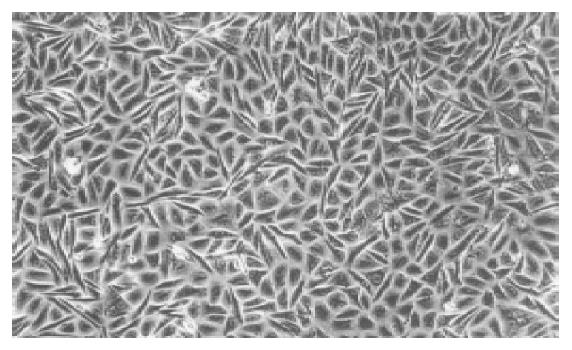
Under a phase-contrast microscope, KKU-100 exhibited compact, monotonous polygonal to spindle cells. The cells were ‘floating’ or ‘clumping’ in a confluent monolayer that could be shaken free, mixed into the medium and transf-erred to another flask (Figure 1). Individual KKU-100 cells had a large nucleus containing 2-5 nucleoli and a clear cytoplasm.
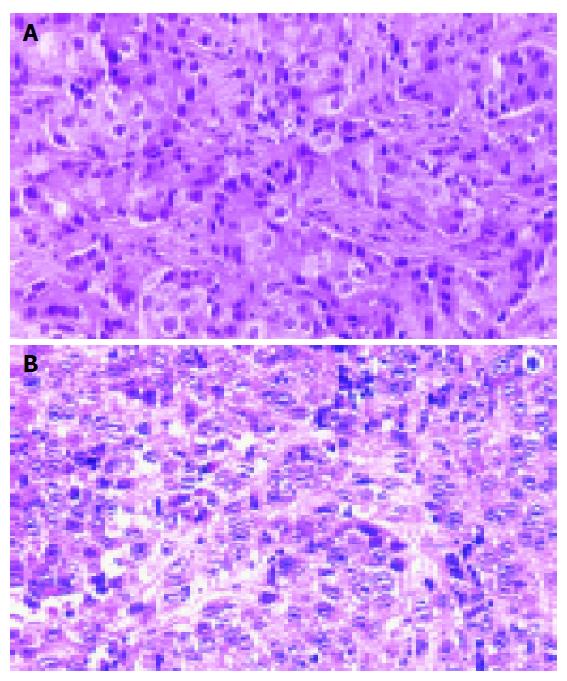
Tumor nodules were developed in the nude mice three weeks after inoculation of KKU-100 cells and reached their greatest dimension of 1.6 cm in 10 wk. Serial transplantations into other nude mice had a shorter induction time for tumor nodules and grew larger. The second generation of transplanted tumors developed one week post inoculation and reached a maximum size of 4.4 cm in 10 wk. Histologically, the transplanted tumor was a poorly differentiated adenocarcinoma, similar to the primary tumor (Figures 2A and 2B).
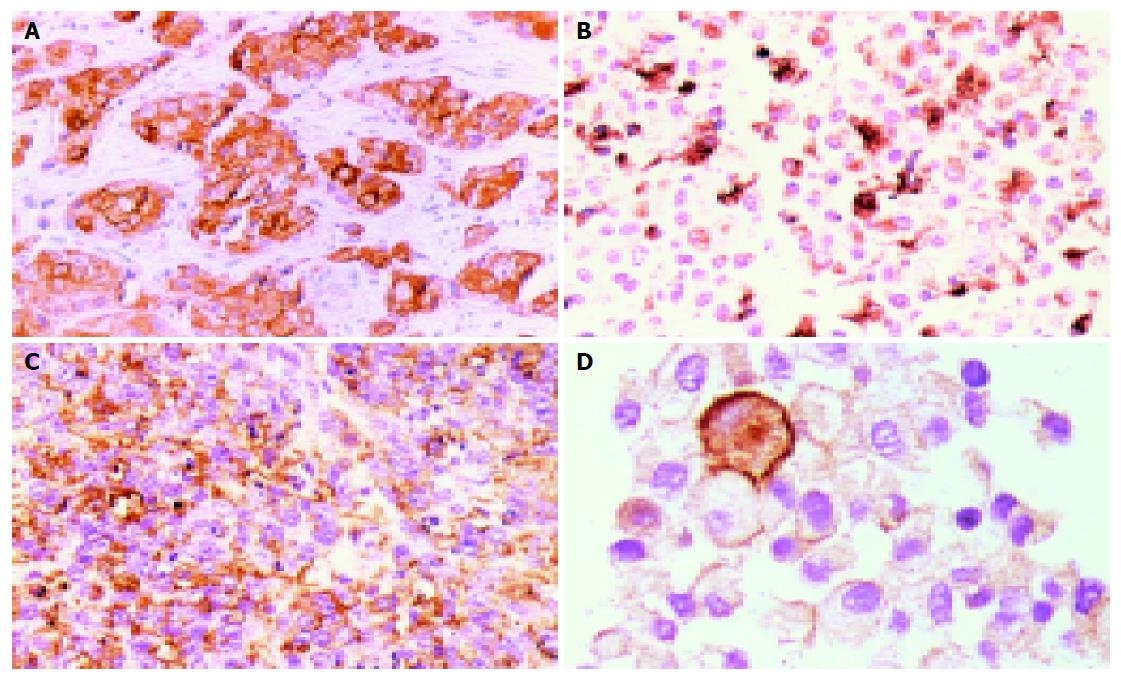
Expression and grading of cellular antigens, tumor markers and cancer gene products of the original tumor and KKU-100 cells are shown in Table 1. KKU-100 cells expressed cytokeratin, EMA, CEA, and CA125 but not AFP, CA19-9, desmin, c-met and p53. These protein expressions were similar to those of the primary tumor. However, heterotransplanted tumors in nude mice retained only EMA protein (Figures 3A-3D and Table 1).
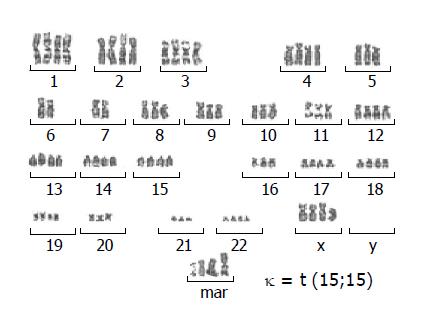
A cytogenetics study revealed a number of chromosomes ranging between 56 and 92 with a modal chromosome number of 78. The G-banded analysis demonstrated aneuploidy karyotype with marked chromosomal structural abnormalities. Several marker chromosomes were noted. Example of the karyotype is shown in Figure 4.
Mycoplasmas were not detected in the spent medium during cell culturing.
A small number of cholangiocarcinoma cell lines are available for cancer research and little is known about this bile duct cancer. To date, approximately eight cell lines have been developed from intrahepatic bile duct cancer, namely HChol-Y1[5], SNU-1079[9], HuCC-T1[11], PCI:SG231[12], CC-SW-1 and CC-LP-1[13], HuCCA-1[14], KMC-1[16]. There is no cell line, to our knowledge, developed from porta hepatis, though tumors at this site are lethal and account for about two-thirds of all cholangiocarcinomas[17,18]. This is probably due to the difficulty of tumor resection and obtaining specimens[19]. KKU-100 may probably be the first porta hepatic-derived and egg-proven Opisthorchis-associated cholangiocarcinoma cell line. A previous cholangiocarcinoma cell line associated with opisthorchiasis, detected by ELISA, was isolated from a Thai patient[14]. Our cell line is the second cell line developed in the area endemic for opisthorchiasis and cholangiocarcinoma in Thailand.
KKU-100 was derived from poorly differentiated tubular adenocarcinoma, the common histologic type of cholang-iocarcinoma reported in Thailand[20]. It possesses some characteristics of carcinoma in nature as evidenced by the expression of cytokeratin and EMA but not desmin. An electron microscopic study revealed microvilli and junctional complexes, the distinguishing ultrastructural features of adenocarcinoma (data not shown). Heterotransplantation of the KKU-100 cells into nude mice retained its histological features of the primary tumor. This is similar to several other cholangiocarcinoma cell lines that produce a similar histologic type in heterotransplanted animals[8,10,12,13]. However, some immunocytochemical characteristics of the cell line were lost such as cytokeratin, CEA and CA125 upon transplantation. This may be due to the natural selection and adaptation of the cells to survive under culture condition or nude mouse microenvironment[21,22].
Immunocytochemical analysis of tumor markers revealed that KKU-100 was positive for CEA, CA125 but not AFP and CA19-9-corresponding to the original tumor. The expression of CEA but not AFP in the primary tumor tissue and cells correlated with a marked elevation of serum CEA (44.6 ng/mL) and negative for AFP in this patient (data not shown). This is common in pure cholangiocarcinoma, where elevated levels of serum CEA occur in ≤80% of cases[23,24], whereas AFP is almost negative. However, the expression of CEA and CA 125 in KKU-100 cells was less than the primary tumor. This is similar to most established cholangiocarcinoma cell lines that show minimal or no CEA expression[6,8,13]. The lowering or loss of some protein expression may be from the natural selection and adaptation of the cells to environment as previously mentioned. Only one cholangiocarcinoma cell line, HuCCA-1, exhibits CA 125[14], our cell line is the second. In contrast, several cholangiocarcinoma cell lines can express or secrete CA19-9, such as HChol-Y1[10], HuCC-T1[11], KMBC[8] and KMC-1[16]. Other tumor markers secreted/expressed by KKU-100 will be investigated for use in diagnostics and early detection in endemic areas.
Alterations of oncogenes and tumor suppressor genes are involved in the malignant transformation and progression of nearly all tumors. For cholangiocarcinoma, however, little is known about its molecular carcinogenesis. The p53 tumor suppressor gene, a common genetic alteration in various cancers, is over-expressed in up to 78.5% of cholangioc-arcinomas[25-27], while mutations of exon 5-8 range between 5% and 37.5%[28-30]. Overexpression of c-met oncogene products has been described in about 60% of human chola-ngiocarcinomas[31] and in furan-induced cholangiocarcinomas in rats[32]. However, KKU-100 cells show no expression of these two gene products. Besides the two genes studied, several other gene alterations may occur in KKU-100 because there are marked chromosomal abnormalities in both number and structures. Detailed studies are in progress.
Cholangiocarcinoma is a major concern in Northeast Thailand because it is a fatal, malignant neoplasm with no curative treatment, neither chemotherapy nor radiation[18]. At present, only surgical excision of the detectable tumor is associated with any improved survival[18]. Since most patients have already advanced stage cancer when they arrive, palliative treatment is all that can be offered[4]. Further study on the various aspects of cholangiocarcinoma, such as tumor biology, cellular and molecular carcinogenesis, biomarkers for early diagnosis and drug responses to new therapeutic agents, is needed. KKU-100 cells should prove a valuable aid to such research and applicable management of cholangiocarcinoma.
The authors thank Ratana Thatarporn, Erika Kuroichi and Suvit Balthaisong for technical assistance, and Mr. Bryan Roderick Hamman for helping with the English-language presentation.
| 1. | Vatanasapt V, Uttaravichien T, Mairiang EO, Pairojkul C, Chartbanchachai W, Haswell-Elkins M. Cholangiocarcinoma in north-east Thailand. Lancet. 1990;335:116-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Jongsuksuntigul P, Imsomboon T. Opisthorchiasis control in Thailand. Acta Trop. 2003;88:229-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 184] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 3. | Infection with liver flukes (Opisthorchis viverrini, Opisthorchis felineus and Clonorchis sinensis). IARC Monogr Eval Carcinog Risks Hum. 1994;61:121-175. [PubMed] |
| 4. | Vatanasapt V, Sripa B, Sithithaworn P, Mairiang P. Liver flukes and liver cancer. Cancer Surv. 1999;33:313-343. |
| 5. | Hudd C, Euhus DM, LaRegina MC, Herbold DR, Palmer DC, Johnson FE. Effect of cholecystokinin on human cholangiocarcinoma xenografted into nude mice. Cancer Res. 1985;45:1372-1377. [PubMed] |
| 6. | Knuth A, Gabbert H, Dippold W, Klein O, Sachsse W, Bitter-Suermann D, Prellwitz W, Meyer zum Büschenfelde KH. Biliary adenocarcinoma. Characterisation of three new human tumor cell lines. J Hepatol. 1985;1:579-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 177] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Katoh H, Shinbo T, Otagiri H, Saitoh M, Saitoh T, Ishizawa S, Shimizu T, Satoh A, Tazawa K, Fujimaki M. Character of a human cholangiocarcinoma CHGS, serially transplanted to nude mice. Hum Cell. 1988;1:101-105. [PubMed] |
| 8. | Yano H, Maruiwa M, Iemura A, Mizoguchi A, Kojiro M. Establishment and characterization of a new human extrahepatic bile duct carcinoma cell line (KMBC). Cancer. 1992;69:1664-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Ku JL, Yoon KA, Kim IJ, Kim WH, Jang JY, Suh KS, Kim SW, Park YH, Hwang JH, Yoon YB. Establishment and characterisation of six human biliary tract cancer cell lines. Br J Cancer. 2002;87:187-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Yamaguchi N, Morioka H, Ohkura H, Hirohashi S, Kawai K. Establishment and characterization of the human cholangiocarcinoma cell line HChol-Y1 in a serum-free, chemically defined medium. J Natl Cancer Inst. 1985;75:29-35. [PubMed] |
| 11. | Miyagiwa M, Ichida T, Tokiwa T, Sato J, Sasaki H. A new human cholangiocellular carcinoma cell line (HuCC-T1) producing carbohydrate antigen 19/9 in serum-free medium. In Vitro Cell Dev Biol. 1989;25:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 119] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Storto PD, Saidman SL, Demetris AJ, Letessier E, Whiteside TL, Gollin SM. Chromosomal breakpoints in cholangiocarcinoma cell lines. Genes Chromosomes Cancer. 1990;2:300-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Shimizu Y, Demetris AJ, Gollin SM, Storto PD, Bedford HM, Altarac S, Iwatsuki S, Herberman RB, Whiteside TL. Two new human cholangiocarcinoma cell lines and their cytogenetics and responses to growth factors, hormones, cytokines or immunologic effector cells. Int J Cancer. 1992;52:252-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 78] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Sirisinha S, Tengchaisri T, Boonpucknavig S, Prempracha N, Ratanarapee S, Pausawasdi A. Establishment and characterization of a cholangiocarcinoma cell line from a Thai patient with intrahepatic bile duct cancer. Asian Pac J Allergy Immunol. 1991;9:153-157. [PubMed] |
| 15. | Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10193] [Cited by in RCA: 10458] [Article Influence: 232.4] [Reference Citation Analysis (0)] |
| 16. | Iemura A, Maruiwa M, Yano H, Kojiro M. A new human cholangiocellular carcinoma cell line (KMC-1). J Hepatol. 1992;15:288-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Uttaravichen T, Buddhisawasdi V, Pairojkul C. Bile duct cancer and the liver fluke. Pathology, presentation and surgical management. Asian J Surg. 1996;19:267-270. |
| 18. | de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med. 1999;341:1368-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 687] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 19. | Ahrendt SA, Cameron JL, Pitt HA. Current management of patients with perihilar cholangiocarcinoma. Adv Surg. 1996;30:427-452. [PubMed] |
| 20. | Sinawat P, Hemsrichart V. A histopathologic study of 61 cases of peripheral intrahepatic cholangiocarcinoma. J Med Assoc Thai. 1991;74:448-453. [PubMed] |
| 21. | Fogh J. Human tumor lines for cancer research. Cancer Invest. 1986;4:157-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Rubin H. Selected cell and selective microenvironment in neoplastic development. Cancer Res. 2001;61:799-807. [PubMed] |
| 23. | Bates SE, Longo DL. Use of serum tumor markers in cancer diagnosis and management. Semin Oncol. 1987;14:102-138. [PubMed] |
| 24. | Jalanko H, Kuusela P, Roberts P, Sipponen P, Haglund CA, Mäkelä O. Comparison of a new tumour marker, CA 19-9, with alpha-fetoprotein and carcinoembryonic antigen in patients with upper gastrointestinal diseases. J Clin Pathol. 1984;37:218-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 127] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Ohashi K, Nakajima Y, Kanehiro H, Tsutsumi M, Taki J, Aomatsu Y, Yoshimura A, Ko S, Kin T, Yagura K. Ki-ras mutations and p53 protein expressions in intrahepatic cholangiocarcinomas: relation to gross tumor morphology. Gastroenterology. 1995;109:1612-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 94] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Rizzi PM, Ryder SD, Portmann B, Ramage JK, Naoumov NV, Williams R. p53 Protein overexpression in cholangiocarcinoma arising in primary sclerosing cholangitis. Gut. 1996;38:265-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Terada T, Nakanuma Y. Expression of apoptosis, proliferating cell nuclear antigen, and apoptosis-related antigens (bcl-2, c-myc, Fas, Lewis(y) and p53) in human cholangiocarcinomas and hepatocellular carcinomas. Pathol Int. 1996;46:764-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Kiba T, Tsuda H, Pairojkul C, Inoue S, Sugimura T, Hirohashi S. Mutations of the p53 tumor suppressor gene and the ras gene family in intrahepatic cholangiocellular carcinomas in Japan and Thailand. Mol Carcinog. 1993;8:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Sturm PD, Baas IO, Clement MJ, Nakeeb A, Johan G, Offerhaus A, Hruban RH, Pitt HA. Alterations of the p53 tumor-suppressor gene and K-ras oncogene in perihilar cholangiocarcinomas from a high-incidence area. Int J Cancer. 1998;78:695-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 30. | Petmitr S, Pinlaor S, Thousungnoen A, Karalak A, Migasena P. K-ras oncogene and p53 gene mutations in cholangiocarcinoma from Thai patients. Southeast Asian J Trop Med Public Health. 1998;29:71-75. [PubMed] |
| 31. | Terada T, Nakanuma Y, Sirica AE. Immunohistochemical demonstration of MET overexpression in human intrahepatic cholangiocarcinoma and in hepatolithiasis. Hum Pathol. 1998;29:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 114] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Radaeva S, Ferreira-Gonzalez A, Sirica AE. Overexpression of C-NEU and C-MET during rat liver cholangiocarcinogenesis: A link between biliary intestinal metaplasia and mucin-producing cholangiocarcinoma. Hepatology. 1999;29:1453-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
Science Editor Wang XL Language Editor Elsevier HK