INTRODUCTION
The multidrug resistance (MDR), which develops in tumor cells, is a major obstacle to the success of cancer chemotherapy[1,2]. P-glycoprotein (p-gp) is a transmembrane protein that acts as an ATP-dependent efflux pump to remove drugs from cells. It is encoded by the mdr1 gene and has been shown to increase with the development of MDR[3]. Up to now, there are at least six members of mdr1, MRP, TopoIIα, GSTp1, MGMT and LRP in MDR family[4-8].
MDR models used currently are basically induced ex vivo and do not necessarily reflect living (in vivo) tumors accurately[9,10]. In the present study, we aimed to set up a nude mice mdr1 model of orthotopic transplantation of liver neoplasm by abdominal chemical therapy at intervals.
Methods including immunohistochemistry, RT-PCR and flow cytometry have been used in the diagnosis of drug resistance[11-13]. They have a common characteristic, which is that a specimen of target cancer tissue is essential. These methods are not useful in identifying non-resective liver neoplasms. A method that is convenient, rapid and overcoming these limitations would be advantageous in clinical assessment. Sestamibi is a cationic lipophilic agent, widely used for myocardial perfusion imaging to detect various tumors[14-16]. Recent evidence has shown that sestamibi is a suitable transport substrate for p-gp and could provide additional information about the p-gp status of tumor cells[17,18]. So we detected the level of p-gp protein by Tc-99m sestamibi on the nude mice models to evaluate the feasibility of this new way.
MATERIALS AND METHODS
Reagents and drugs
DMEM and TRIzol reagent (Gibco, USA), Pharmorubicin (Pharmacia, USA), M-MLV reverse transcriptase and oligo(dT)15 primer (Promega, USA), Taq DNA polymerase (Biostar, USA), 100-bp DNA ladder (SABC, China), monoclonal antibody JSB-1 (Wuhan Boster Biological Technology Co., Ltd, China) and HistostainTM-Plus kit (Beijing Zhongshan Biotechnology Co., Ltd, China) were obtained. Mdr1 primers and β-actin primers were designed by using GeneFisher1.3 and produced by TaKaRa (Japan).
Establishment of nude mice model
HepG2 cells were injected subcutaneously at the back of each nude mouse at a concentration of 5×106 cells/0.1 mL to establish a model of tumor-bearing mice. Then, the orthotopic mdr1 model was established by implanting tumor bits under the envelope of the mice liver and inducing with abdominal chemotherapy using Pharmorubicin.
Inducing tumor
Ten days post-operatively, when the diameter of the tumor reached to 0.3-0.5 cm, the nude mice were grouped randomly into an inducing group (n = 20) and a control group (n = 5). Each mouse was injected with Pharmorubicin (10 µg/g ip, twice per week, for 6 wk). Mice in the control group were injected with saline.
Anatomy and pathology
Physical examination, ultrasonography, spiral CT, and visual inspection were used to examine the progression of the tumor. Tumor tissues were fixed with 10% neutral formaldehyde, then paraffin-embedded and sections were made. Following dissection of the mice, the size, number, form, and metastasis of the tumor were carefully observed.
RT-PCR analysis of mdr1
Mdr1 primer (174 bp): 5’-CAT TGG TGT GGT GAG TCA GG-3’ and 5’-CTC TCT CTC CAA CCA GGG TG-3’; β-actin primer (247 bp): 5’-CCC AGA GCA AGA GAG GCA TC-3’ and 5’-AGC ACA GCC TGG ATA GCA AC-3’.
Total RNA was isolated using the TRIzol reagent according to the manufacturer’s instructions and reverse transcribed. cDNA was amplified in a 50 μL reaction containing 10×buffer (5.0 μL), cDNA (2.0 μg), primers (each 1.0 μL), 25 mmol/L MgCl2 (3.0 μL), 10 mmol/L dNTPs (1.0 μL), and Taq polymerase (2.5 U). Each of total 30 PCR amplification cycles consisted of denaturation at 94 °C for 1 min, primer annealing at 57 °C for 45 s, extension at 72 °C for 45 s, for a total of 30 cycles. The PCR products were separated by electrophoresis on 2% agarose gels.
Determination of p-gp protein expression level
Tumor tissues were fixed with 10% neutral formaldehyde, then paraffin embedded and sections were made. The sections were stained with the monoclonal antibody JSB-1 followed by Streptavidin-Peroxidase kit (SP kit).
Tc-99m sestamibi SPECT
After administration of 7 MBq Tc-99m sestamibi (prepared according to the manufacturer’s instructions), images were obtained at 20-min intervals. Planar abdominal images were acquired on 256×256 matrices using a low-energy high-resolution collimator. The liver/heart ratio was calculated by ROI (regions of interest) technique.
Image and statistical analysis
Image analysis was performed by using ImageTool 2.0 and statistical analysis by SAS 8.01. The Student’s t test was performed to evaluate the significant differences between groups. Significance was determined at P<0.01.
RESULTS
Establishment of nude mice model
The implantation operation took approximately 12 min to complete. After 30 min, the mice became conscious and returned to normal activity by 60 min with a mortality rate of 0% (0/25). Successful tumor implantation rate was 90% (22/25), and the success rate of a second time implantation was 100% (3/3). Tumor induced successful rate was 80% (16/20).
Anatomy and pathology
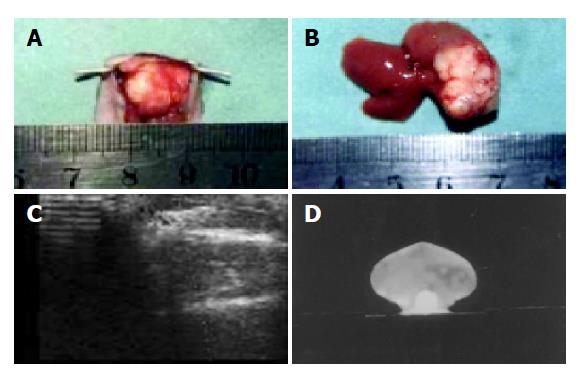
The tumors were multilobular, hard, limited in one lobe of liver with an intact envelope. The tumors were clearly delimited from adjacent liver tissue but also appeared with abdominal metastasis (Figures 1A and 1B). Tumors of a diameter of approximately 0.5 cm could be detected by ultrasound and CT examination. The tumor manifests as a low-density echo by ultrasound and color Doppler flow imaging shows a developed artery spectrum. CT identified the tumors as a low-density lesion after reinforcement (Figures 1C and 1D). The pathological section showed that the tumor cells were heterogeneous in size and manifest karyomegaly. The nucleus was deeply dyed and appeared heterogeneous.
Figure 1 The specimen and images by Doppler and CT.
A: Specimen of tumor model after 15 d of implantation; B: specimen of tumor model after 25 d of implantation; C: color Doppler image of tumor model after 25 d of implantation; D: contrast-enhanced abdominal CT image of tumor model after 20 d of implantation.
Expression of mdr1
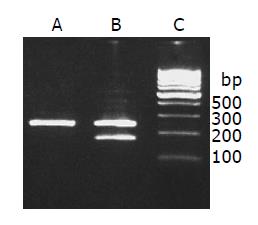
RT-PCR assay demonstrated that the mdr1 mRNA expression was significantly stronger in the induced group than in the control group. The relative density of each band was scanned and expressed relative to β-actin. Expression of mdr1 in the induced group was 23-fold higher than that in the control group (56.11±5.82% induced vs 2.44±0.18% control group, t = 20.4, P<0.01) (Figure 2).
Figure 2 Mdr1 mRNA expression.
lane A: Control group; lane B: induced group; lane C: 100-bp marker.
Immuohistochemistry
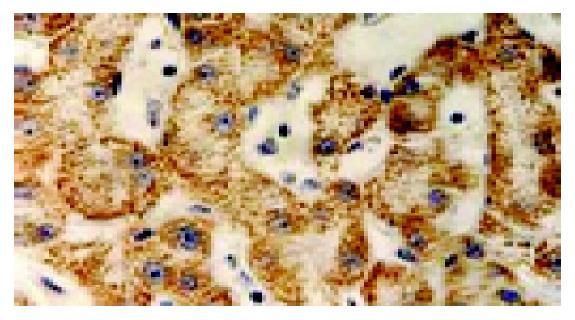
Immuohistochemical staining indicated that there was a significant expression of p-gp in the inducing group, with thick and buffy particles in the cell membrane and cytoplasm compared to the control group. The positive cells exhibited a nest distribution. P-gp protein expression in the induced group is 13-fold higher than that of the control group (t = 24.9, P<0.01, Figure 3).
Figure 3 Expression of p-gp by using immunohistochemistry (SABC ×200).
Tc-99m sestamibi SPECT
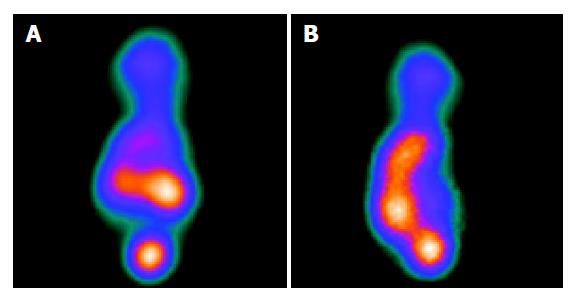
The MIBI SPECT of the induced group showed a radioactivity deficiency in the tumor which contrasted with a radiographic dense area in the control group. The liver/heart ratio was significantly decreased in the induced group as compared to the control group. The average liver/heart ratio was 0.68±0.10 in the induced group and 1.86±0.32 in the control group (P<0.01, Figures 4A and 4B).
Figure 4 SPECT images of the nude mice model.
A: SPECT of control group showing dense radioactivity; B: SPECT of control group showing deficiency radioactivity.
DISCUSSION
MDR of tumor cells is a major obstacle to the success of cancer chemotherapy[1,2]. A recent focus has been the recognition of the phenomenon of increased expression of p-gp protein encoded by the mdr1 gene, which is increased in liver neoplasms[19,20]. P-gp is a 170-ku membrane glycoprotein, which acts as an energy-dependent efflux pump exporting a variety of structurally and functionally unrelated compounds, including cytotoxic drugs, calcium channel blockers, calmodulin inhibitors, and peptides[21,22]. The importance of p-gp as a mechanism of drug resistance in cancer cells has been well characterized. However, many other associated genes have also been identified, such as MRP, TopoIIα, GSTp1, MGMT, LRP among others. The mechanisms of MDR induced by these other proteins is reportedly different from p-gp[4-8]. It has been reported previously that p-gp is increased significantly in liver neoplasms[23-25].
Methods including immunohistochemistry, molecular biology (RT-PCR) and flow cytometry have been used in the diagnosis of drug resistance[11-13]. They have a common characteristic, which is that a specimen of target cancer tissue is essential. These methods are not useful in identifying non-resective liver neoplasms. A method that is convenient, rapid and overcoming these limitations would be advantageous in clinical assessment.
Previously, we used SMMC7721 MDR cells to assess drug resistance. The limitations were the length of time required for testing, and the trial-and-error methodology, which is problematic. Most of all, the cells are induced ex vivo, which is clearly different from the situation in clinical cancers. Establishment of a nude mice model of orthotopic liver neoplasm using abdominal chemotherapy would ideally represent the in vivo development of MDR of liver cancers. In the present study, we demonstrated that such a model is possible, with an inducing successful rate of 80% (16/20). The mdr1 mRNA expression of the induced group was 23 times that of the control group and p-gp protein expression was 13-fold the control group.
Tc-99m sestamibi is a cationic lipophilic agent developed for myocardial perfusion imaging. It is also used for diagnostic imaging of various tumors. Tc-99m sestamibi has been shown to be a substrate for p-gp, and accumulation of Tc-99m sestamibi in MDR cells is decreased or null. The washout rate of Tc-99m in tumor cells is determined by the quality of sestamibi in cells[26]. It has been reported that the MIBI SPECT in breast tumors could be useful in evaluating the level of p-gp expression[27-31]. Our present study demonstrates that, in the nude mice model of orthotopic liver neoplasm, the density of the sestamibi image in the tumors of the induced group is sparse (liver/heart: 0.68±0.10), while the sestamibi density in tumors of the control group was much higher (liver/heart: 1.86±0.32). This observation indicates the potential use of Tc-99m sestamibi as a p-gp probe for in vivo determination of p-gp transport function.
In conclusion, we have successfully established the nude mice mdr1 model of orthotopic liver neoplasm, and demonstrated the usefulness of SPECT by Tc-99m sestamibi for diagnosis of expression of p-gp. This model is representative of the clinical situations and could be of potential importance in the researches about the diagnosis and reversal of MDR.