Published online Jun 7, 2005. doi: 10.3748/wjg.v11.i21.3300
Revised: March 4, 2004
Accepted: April 5, 2004
Published online: June 7, 2005
AIM: To construct fusion protein of a single-chain antibody (scFv) against transferrin receptor (TfR) with alkaline phosphatase (AP).
METHODS: The VH-linker-VL, namely scFv gene, was prepared by amplifying the VH and VL genes from plasmid pGEM-T-VH and pGEM-T-VL with splicing overlap extension polymerase chain reaction (SOE PCR). After the ScFv gene was modified by Sfi I and Not I, it was subcloned into the secretory expression vector pUC19/119, and then was transformed into E.coli TG1. The positive colonies were screened by colony PCR and their expressions were induced by IPTG. ScFv gene was gained by digesting ScFv expression vector pUC19/119 with Sfi I and Not I restriction enzymes, then subcloned into expression vector pDAP2, followed by transformation in E.coli TG1. The positive colonies were selected by bacterial colony PCR. The expression of fusion protein (scFv-AP) was induced by IPTG. Its activity was detected by enzyme immunoassay. The molecular weights of scFv and scFv-AP were measured by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
RESULTS: The product of SOE PCR formed a band of 700 bp in agarose gel electrophoresis. SDS-PAGE demonstrated the molecular weight of scFv was 27 ku. Immunofluorescent assay (IFA) demonstrated its reactivity with TfR. The molecular weight of scFv-AP was 75 ku. Enzyme immunoassay showed that scFv-AP could specifically bind to human TfR and play AP activity.
CONCLUSION: We have successfully prepared the anti-human TfR scFv and constructed the fusion protein of scFv and AP. It is promising for immunological experiments.
- Citation: Yang DF, Zhu HF, Wang ZH, Shen GX, Tian DY. Construction of single chain Fv antibody against transferrin receptor and its protein fusion with alkaline phosphatase. World J Gastroenterol 2005; 11(21): 3300-3303
- URL: https://www.wjgnet.com/1007-9327/full/v11/i21/3300.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i21.3300
Transferrin receptor (TfR) is a transmembrane glycoprotein, which expresses on the surface of rapidly proliferative cells and highly expresses in various tumor cells, especially in hepatoma carcinoma cells[1]. It is associated with substance transfer in cell cycles[2-5]. Therefore, anti-TfR antibody (Ab) is regarded as a potential molecule for the located diagnosis and target treatment of tumors[6-8]. In our previous studies, the plasmid expressing VH and VL had been successfully prepared[9]. In this paper, we prepared single chain Fv (scFv) antibody against TfR and then constructed the fusion protein of scFv and alkaline phosphatase (AP) by using gene engineering technique.
E.coli TG1 was purchased from Stratagen Corporation. Clone vector pGEM-T was produced by Promega Co. Ltd. The prokaryotic secretory expression vectors pUC19/119 and pDAP2 were generously presented by Dr. RJ Kerschboumer. The former carries a T7 RNA polymerase promoter, a leading peptide pelB, His and c-myc markers which make for the expression and purification of scFv, and a termination codon. The pelB, His and c-myc markers, and termination codon can be cut during scFv preparation. The latter contains AP gene and Lac promotor which switches on the expression of fusion protein scFv-AP under the induction of isopropyl β-D-thiogalactopyanoside (IPTG). Its N-terminal pelB leader can lead the fusion protein into the periplasma and its hexa-histidine-tag makes it easy to be purified via affinity chromatography[10].
T4 DNA ligase was purchased from USB Corporation (Cleveland, USA), calf AP and RNase from Boerhinger-Mannheim Biochemicals (Indianapolis, USA), endonucleases SfiI and NotI from Jingmei Biotech Co., Ltd.
A primer was designed according to the complementary sequences of FR1 region of VH, which contained cleavage sites of SfiI: 15560, VH2 SfiI 5’-TATGCGGCCCAGCC-GGCCATGGC(A/C)(G/C)AG(G/A)T(T/C)CAGC-TGCAGCA-3’; Another primer was designed according to the sequences of FR4 of VH, which contained 2/3 sequences of 5’-terminal glycine linker [(Gly4Ser)3]: 15 561,VH3 Linker, 5’-GGAGCCGCCGCCGCCAGAACC-ACCACCACC(C/T)G(A/C)(T/G)GAGAC(T/A)GTGA(G/C)-3’.
A pair of primers for VL were designed according to the same segments, the forward primer contained 2/3 sequences of 3’-terminal glycine linker and reverse primer contained Not I cleavage site: 15 562, VL5 Linker, 5’-GGCGGCGGCGGCTCCGGTGGTGGTGGTTCT(G/C)A(C/A)ATTGT(G/C)(A/C)T(G/C)ACCC-3’; and 1261K, VL Not I, 5’-CGGGCGGCCGCTTTGATT-TCCAGC TTGGTCCC-3’.
VH gene of monoclonal antibody (McAb) was amplified from pGEM-T-VH by PCR with primers 15560 and 15561. VL gene was amplified from plasmid pGEM-T-VL with primers 15562 and 1261 K. VH-Linker-VL (scFv gene) was obtained by splicing overlap extension PCR (SOE PCR). Cleaved by Sfi I and Not I, ScFv gene was subcloned into vector pUC19/119, which was then transformed into competent E.coli TG1, and positive colonies were selected by colony PCR. The expression of scFv was induced by IPTG and its molecular weight was identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Human TfR expressing cell line SMMC7721 at logarithmic phase was plated into 96-well plates (2×105/well), then IPTG induced supernatant was added to the wells (100 μL/well). After incubated for 30 min at 4 °C, rabbit anti-His McAb was added and indirect immunofluorescent assay (IFA) was performed to identify the antibody activity.
The plasmid pUC19/119 expressing scFv was digested by Sfi I and Not I to produce scFv gene, then the gene was subcloned into vector pDAP2 to construct the gene encoding fusion protein (scFv-AP) directly. The expression vector was transformed into E.coli TG1. Positive colonies were screened by colony PCR, and the expression of scFv-AP was induced by IPTG. The molecular weight was measured by SDS-PAGE. SMMC7721 cells at 1×107/mL at logarithmic phase were plated into 96-well plates (20 μL/well), glutaraldehyde 20 μL/well was added to fix cells for 2 min. After washed twice by PBS, IPTG induced cultural supernatant was added to the wells (100 μL/well) and incubated at 37 °C for 60 min. The plate was washed thrice by PBS, the substrate was added and then the A value was measured at 405 nm.
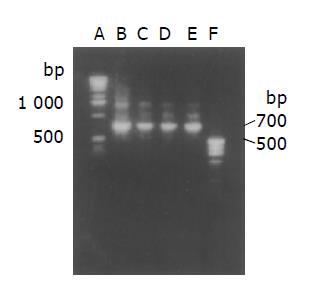
On agarose gel electrophoresis, SOE PCR product formed a single band of expected size of 700 bp (Figure 1).
After collection, purification and quantification, the SOE PCR product was digested by SfiI and NotI, and linked with pUC19/119. The conjugate was transformed into E.coli TG1 on Amp+ LB plates. Ten colonies were identified from 20-30 colonies by colony PCR for selection of positive colonies. After two positive colonies were proliferated by culture, small quantities of plasmids were extracted and were put through plasmid PCR. On electrophoresis, PCR product formed a band of 700 bp (Figure 2). The plasmid extracted from positive colonies was sequenced and confirmed that the prepared sequence was scFv gene (VH-Linker-VL, data not shown).
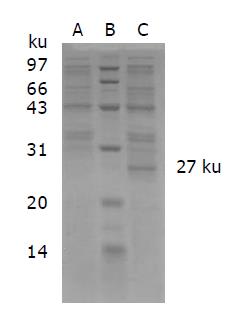
The positively transformed bacteria of scFv gene were induced by IPTG; the bacteria were cleaved and examined by SDS-PAGE. On the gel, a specific protein band was displayed at 27 ku. The control bacteria did not display this band (Figure 3). IFA showed that SMMC7721 cells reacted with IPTG-induced cultural supernatant. Negative control did not show membrane fluorescence as it reacted with SMMC7721 cells. It suggested that IPTG-induced cultural supernatant contained anti-human TfR scFv.
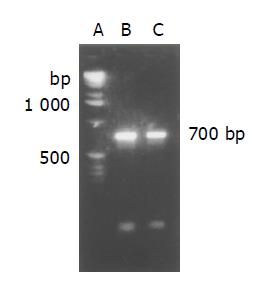
After the plasmid pUC19/119 which carries anti-TfR scFv gene and vector pDAP2 was digested by SfiI and NotI, ScFv gene was converted into vector pDAP2 with AP gene. The conjugate was transformed into E.coli TG1 on Amp+ LB plates. Twenty to thirty colonies were obtained, 10 positive colonies were determined by colony PCR and agarose gel electrophoresis. The positive colonies were cultured and proliferated, and the plasmid was extracted and underwent plasmid PCR. Its product formed a band of 700 bp on electrophoresis (Figure 4). It suggested that the scFv gene had been subcloned into the vector pDAP2.
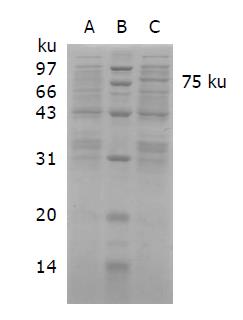
ScFv-AP gene transformed bacteria were cultured overnight, and induced by IPTG. SDS-PAGE was performed to determine the molecular weight of the expression product. On the gel, the product formed a specific protein band of 75 ku. It is consistent with the molecular weight of fusion protein. However, the empty bacteria did not display the protein band at 75 ku (Figure 5). It suggested that the fusion protein of anti-TfR scFv-AP had been successfully constructed and expressed.
The supernatant from scFv-AP positive bacteria was added into SMMC7721 cell coated plates, and then the substrate of AP was added into the wells to stain. The average A value of positive wells was 1.32, obviously higher than that of control wells (0.32). It suggested that IPTG induced cultural supernatant contained the specific fusion proteins, which had a specific binding reactivity with human TfR and possessed the activity of AP.
In general, TfR predominantly expresses on the surfaces of proliferative cells, especially malignant cells. Therefore, detection of TfR is useful to diagnosis of tumors. A report on 20 surgically resected samples of HCC showed that TfR expressed highly in cancer tissue, whereas expressed very low in pericancer tissues and normal tissues[11]. Its importance for cancer diagnosis and prognostic evaluation has been also documented in leukemia[4], lymphoma[5], pulmonary cancer[12,13], breast cancer[14], and gastroenteric cancer[15]. There is an additional kind of transferrin receptor in serum, termed as soluble transferrin receptor (sTfR). It is lack of transmembrane and intracellular segment of TfR and associated with iron metabolism. The detection of sTfR is useful for adjuvant diagnosis of iron deficiency anemia[16]. sTfR represents a valuable quantitative assay of marrow erythropoietic activity as well as a marker of tissue iron deficiency[17].
Immunoassay is the major measurement method of TfR. McAb and polyclonal serum are employed for this test. ScFv is a small single-strand peptide that is formed by linking VH and VL of immunoglobulin. Fv segment comprising VH and VL is a tiny unit of an antibody molecule. ScFv has many advantages[18,19]: (1) Its small molecule, only 1/6 of the full antibody, makes it potentially applicable in the clinical diagnosis and treatment of both infectious diseases and cancers due to its good penetration and rapid clearance; (2) It can be mass-produced by fermentation in E.coli; (3) Its single chain makes it easy to fuse with other proteins or toxins[20]. In this paper, anti-TfR scFv was successfully constructed and exhibited a good specificity and sensitivity. It has potential to substitute McAb for immunoassay of TfR, especially for the immunohistochemistry assay.
In ordinary EIA and IFA, it is necessary to label the anti-Ab1 antibody (Ab2) with a kind of enzyme or fluorescein. In the protocol, every batch of labeled antibody needs to be standardized. The fusion protein for special purpose produced by gene engineering technique can replace the routine chemistry linking enzyme-labeled antibody and can be mass-produced. ScFv-enzyme fusion protein can specifically bind to the antigen and catalyze the substrate reaction. Compared to McAb, the small size of Fv fragment and the absence of the constant regions of the antibody polypeptide chain suggest fewer background reactions. It shows a unique advantage as a reagent for immunoassay and antigen location. The application of scFv-enzyme fusion protein in immunological test will omit the procedure of enzyme labeling or fluorescence labeling. For example, the fusion protein of AP and scFv against tumor associated antigens (e.g., CEA or AFP) can display the AP activity as well as the binding ability to CEA or AFP. With this detection system, the detection of CEA or AFP can be finished in a single step. In this report, anti-TfR scFv-AP was successfully constructed, and expressing product could specifically bind to TfR and displayed AP activity. The use of this detection system in immunoassay can omit the step adding the enzyme-labeled antibody. It is promising for immunological experiments and clinical application.
| 1. | Miller YE, Jones C, Scoggin C, Morse H, Seligman P. Chromosome 3q (22-ter) encodes the human transferrin receptor. Am J Hum Genet. 1983;35:573-583. [PubMed] |
| 2. | Shionoya K. A study of relationship between proliferative activity and expressed transferrin receptor content in cancer cells. Kokubyo Gakkai Zasshi. 1994;61:580-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Högemann-Savellano D, Bos E, Blondet C, Sato F, Abe T, Josephson L, Weissleder R, Gaudet J, Sgroi D, Peters PJ. The transferrin receptor: a potential molecular imaging marker for human cancer. Neoplasia. 2003;5:495-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 152] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Kollia P, Samara M, Stamatopoulos K, Belessi C, Stavroyianni N, Tsompanakou A, Athanasiadou A, Vamvakopoulos N, Laoutaris N, Anagnostopoulos A. Molecular evidence for transferrin receptor 2 expression in all FAB subtypes of acute myeloid leukemia. Leuk Res. 2003;27:1101-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Feremans W, Bujan W, Neve P, Delville JP, Schandene L. CD71 phenotype and the value of gallium imaging in lymphomas. Am J Hematol. 1991;36:215-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Xu L, Huang CC, Huang W, Tang WH, Rait A, Yin YZ, Cruz I, Xiang LM, Pirollo KF, Chang EH. Systemic tumor-targeted gene delivery by anti-transferrin receptor scFv-immunoliposomes. Mol Cancer Ther. 2002;1:337-346. [PubMed] |
| 7. | Moura IC, Lepelletier Y, Arnulf B, England P, Baude C, Beaumont C, Bazarbachi A, Benhamou M, Monteiro RC, Hermine O. A neutralizing monoclonal antibody (mAb A24) directed against the transferrin receptor induces apoptosis of tumor T lymphocytes from ATL patients. Blood. 2004;103:1838-1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Yang DF, Wang S, Zhu HF, Shen GX. The anti-tumor effects of an anti-CD71 chimeric antibody in vitro and its distribution in a tumor xenograph model. Chinese German J Clin Oncol. 2002;1:109-112. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 9. | Wang S, Shen GX, Jiang L, Wang XL, Xiong W, Lu CX. Sequence analysis of functional and nonfunctional Vk genes from a hybridoma. Zhongguo Mianyixue Zazhi. 1999;15:82-84. |
| 10. | Kerschbaumer RJ, Hirschl S, Schwager C, Ibl M, Himmler G. pDAP2: a vector for construction of alkaline phosphatase fusion-proteins. Immunotechnology. 1996;2:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Zhou GW, Cai WY, Yang WP, Chen H, Di ZM, Li HW. The study of transferrin receptor gene expression and diagnosis of hepatocellular carcinoma by monoclonal antibody. Shanghai Dier Yike Daxue Xuebao. 2000;20:436-438. |
| 12. | Dowlati A, Loo M, Bury T, Fillet G, Beguin Y. Soluble and cell-associated transferrin receptor in lung cancer. Br J Cancer. 1997;75:1802-1806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Rácz A, Brass N, Heckel D, Pahl S, Remberger K, Meese E. Expression analysis of genes at 3q26-q27 involved in frequent amplification in squamous cell lung carcinoma. Eur J Cancer. 1999;35:641-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Yang DC, Wang F, Elliott RL, Head JF. Expression of transferrin receptor and ferritin H-chain mRNA are associated with clinical and histopathological prognostic indicators in breast cancer. Anticancer Res. 2001;21:541-549. [PubMed] |
| 15. | Shinohara H, Fan D, Ozawa S, Yano S, Van Arsdell M, Viner JL, Beers R, Pastan I, Fidler IJ. Site-specific expression of transferrin receptor by human colon cancer cells directly correlates with eradication by antitransferrin recombinant immunotoxin. Int J Oncol. 2000;17:643-651. [PubMed] |
| 16. | Wang JR, Zou DD. Use of serum transferrin receptor detection in diagnosis of iron deficiency anemia in children. Zhonghua ErKe ZaZhi. 2004;42:388-389. [PubMed] |
| 17. | Beguin Y. Soluble transferrin receptor for the evaluation of erythropoiesis and iron status. Clin Chim Acta. 2003;329:9-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 299] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 18. | Xu L, Tang WH, Huang CC, Alexander W, Xiang LM, Pirollo KF, Rait A, Chang EH. Systemic p53 gene therapy of cancer with immunolipoplexes targeted by anti-transferrin receptor scFv. Mol Med. 2001;7:723-734. [PubMed] |
| 19. | Boado RJ, Ji A, Pardridge WM. Cloning and expression in Pichia pastoris of a genetically engineered single chain antibody against the rat transferrin receptor. J Drug Target. 2000;8:403-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |