Published online Jun 7, 2005. doi: 10.3748/wjg.v11.i21.3264
Revised: June 18, 2004
Accepted: July 27, 2004
Published online: June 7, 2005
AIM: The sphincter of Oddi (SO) plays an important role in delivery of bile into the duodenum. To establish whether vasoactive intestinal polypeptide (VIP) and nitric oxide (NO) were involved in phasic contractile activity of the rabbit SO stimulated by cholecystokinin-octapeptide (CCK-8).
METHODS: Isolated SO muscle rings were cleaned of fat and mounted horizontally on two small L-shaped hooks one of which was connected to a force transducer for the measurement of isometric tension. The experiments were carried out in a thermostatically controlled (37±0.2 °C) organ bath (5 mL) containing Krebs solution. The organ fluid was gassed with 95% O2 and 50 mL/L CO2 to keep the pH at 7.40±0.05. Contractile responses to CCK-8 (1 μmol/L) were evaluated in the presence and absence of NG-nitro-L-arginine (LNNA), an inhibitor of NO synthase (100 μmol/L), and (p-chloro-D-Phe6-Leu17)-VIP (VIPa, 30 μmol/L), a VIP receptor antagonist.
RESULTS: CCK-8 stimulated the phasic activity of the SO. NO synthase inhibition increased the frequency and amplitude of contractions with a slight increase in developed tension. Pre-incubation with VIPa also attenuated this CCK-8 effect. The combined application of LNNA and VIPa abolished the phasic activity of the muscle rings with a marked increase in tension in response to CCK-8.
CONCLUSION: VIP and NO together contribute to an increase in phasic activity of SO.
- Citation: Pálvölgyi A, Sári R, Németh J, Szabolcs A, Nagy I, Hegyi P, Lonovics J, Szilvássy Z. Interplay between nitric oxide and VIP in CCK-8-induced phasic contractile activity in the rabbit sphincter of Oddi. World J Gastroenterol 2005; 11(21): 3264-3266
- URL: https://www.wjgnet.com/1007-9327/full/v11/i21/3264.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i21.3264
The sphincter of Oddi (SO) plays an important role in delivery of bile into the duodenum. In animals like rabbits, opossums and guinea pigs, the partially extraduodenal sphincter operates like a peristaltic pump that actively squeezes bile into the duodenum[1]. Thus, contraction and relaxation mechanisms are of equal importance in controlling normal sphincter function. Regulation of the relaxation function of this sphincter is mainly executed by non-adrenergic, non-cholinergic (NANC) nerves[2]. With regard to the neurotransmitters involved, evidence favors a role for vasoactive intestinal polypeptide (VIP)[2] and nitric oxide (NO) in various species including guinea pigs[3], rabbits[4], and humans[5]. In the rabbit SO the NANC relaxation is completely blocked by NG-nitro-L-arginine methyl ester (L-NAME), an inhibitor of NO-synthase[4]. The inhibitory effect of L-NAME can be reversed by concurrent incubation with L-arginine but not D-arginine indicating the response to be essentially nitrergic. It is widely accepted that NO evokes smooth muscle relaxation through formation of cyclic GMP within muscle cells[6]. Nevertheless, NO has been shown to stimulate the release of VIP from enteric nerve terminals, an effect to enhance or contribute to its ‘per se’ relaxant effect[7].
Much less is known about the influence of endogenous substances that promote the peristaltic pump-like sphincter function through generating phasic contractions. Cholecystokinin (CCK) is generally regarded as the major hormone regulating postprandial SO motility. CCK has been shown to initiate VIP and acetylcholine release at both pre- and post-junctional sites in enteric nervous system through CCKA receptors, whereas CCKB receptors seem to mediate NO release at post-junctional sites[8]. Nevertheless, the contribution of NO and VIP release to CCK-induced stimulation of phasic contractile activity of the SO has not been explored. The present work was therefore concerned with the possibility that an interplay between NO and VIP would contribute a significant degree to CCK-induced phasic contractions in the SO of rabbits.
The experiments in the present work conformed to European Guiding Principles for Care and Use of Experimental Animals. In addition, the experimental protocol applied was approved by the local ethical board of University of Szeged and University of Debrecen, Hungary.
These measurements were described in detail elsewhere[4]. Biliary SO muscle rings of approximately 6 mm in length from adult male New Zealand white rabbits weighing 3500-4000 g were prepared. The papilla Vateri was eliminated and the ampullary part of the muscle rings of approximately 3 mm in length were mounted horizontally on two small L-shaped glass hooks of which one was connected to a force transducer (SG-O2, Experimetria, Budapest, Hungary) attached to a six channel polygraph (R61 6CH, Mikromed, Budapest, Hungary) for measurement and recording of isometric tension as described[4]. One muscle ring was prepared from one animal. The experiments were carried out in an organ bath (5 mL) containing Krebs bicarbonate buffer (mM: NaCl 118.1, KCl 4.7, MgSO4 1.0, KH2PO4 1.0, CaCl2 2.5, NaHCO3 25.0, glucose 11.1) which was maintained at 37 °C and aerated continuously with carbogen (50 mL/L CO2 in oxygen, Ph. Eur. III.). The initial tension was set at 10 mN and the rings were allowed to equilibrate for over an hour. Cholecystokinin octapeptide (CCK-8, 0.01-10 μmol/L), atropine (1 μmol/L), tertrodotoxin (TTX, 1 μmol/L), (p-chloro-D-Phe6, Leu17)-VIP (VIPa, 30 μmol/L), and NG-nitro-L-arginine (LNNA, 100 μmol/L) were added directly to the organ bath in 50-150 μL volume.
The muscle rings underwent brief experimental protocols as follows. Protocol 1: the preparations with a resting tension of 10 mN were exposed to cholecystokinin-octapeptide (CCK-8) (0.1 μmol/L, the EC50 for the peptide in this preparation) subsequent to an equilibration period of over 60 min. After a stable contractile response was obtained, the preparations were washed until tension returned to previous baseline level. Protocols 2 and 3 were to study the effect of atropine and TTX (1 μmol/L for both) on CCK-8-induced increase in phasic activity. Atropine and TTX were applied either prior to CCK-8 or at maximum increase in phasic contractions, respectively. Protocols 4 and 5 were to study the effect of NO synthase inhibition (100 μmol/L LNNA) and neutralization of VIP action on CCK-8-induced responses. The preparations were pre-incubated with either 100 μmol/L LNNA or 30 μmol/L VIPa for over 20 min, and then CCK-8 (0.1 μmol/L) was given. In protocol 6, LNNA and VIPa were applied together prior to CCK-8. Except for protocol 3, baseline contractile patterns were re-gained subsequent to washout; in case of TTX, however, the decrease in baseline contractile amplitudes could not be overcome even by extensive washout.
Atropine, TTX, LNNA, and CCK-8 were obtained from Sigma Chemical Company (St. Louis, USA). Polyclonal VIPa was a generous gift from Joseph Nemeth (Department Pharmacology, Medical University of Pecs). The compounds were dissolved in Krebs solution and added directly to the organ bath except for LNNA which was dissolved in ethanol and then diluted with Krebs solution.
Parameters producing the data for evaluation were as follows. The amplitude of contractions (mN) was referred to as the difference between peak contractions and relaxations. The averages of the amplitudes were calculated for every minute (results are expressed as mean±SE, n = number of freque-ncies in a minute). The frequencies of contractions (cpm) were calculated for every minute. Statistical analysis was performed for every 10 min of the experiments using either Student’s t-test (when the data consisted of two groups) or ANOVA (when three or more data groups were compared). Results were expressed as mean±SE, n = 5, P<0.05 was accepted as statistically significant.
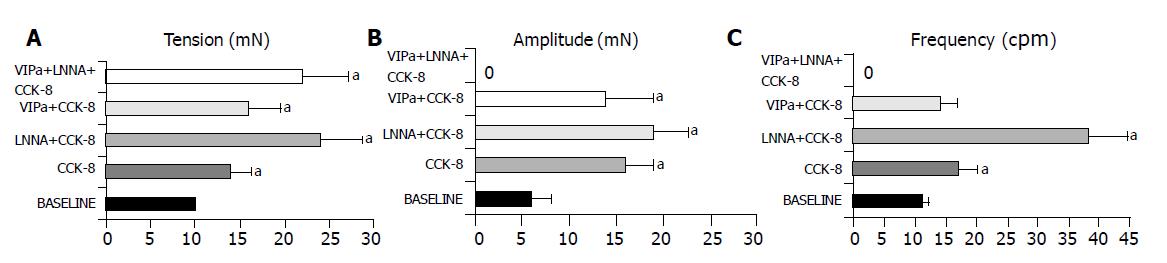
CCK (0.1 μmol/L) increased frequency and amplitude of contractions, and elevated the developed tension of the muscle rings (Figures 1A-1C).
CCK-8-stimulated SO contractions were blocked by TTX (1 μmol/L). Atropine in the same concentration (1 μmol/L) also abolished agonist-induced contractile activity.
NO synthase inhibition by LNNA markedly increased the frequency and amplitude of contractions with a slight increase in developed tension. Pre-incubation with polyclonal VIPa attenuated each CCK-8 effect. Combined application of LNNA and VIPa significantly increased the tension of CCK-8, but abolished the phasic contractile activity of the muscle rings(Figures 1A-1C). Separate application of LNNA and VIPa potentiated the effect of CCK-8-induced phasic contractions, while combined application of these substances completely abolished the phasic activity of the muscle rings with a marked increase in tension in response to CCK-8. These results indicated that VIP and NO together are mediators of the CCK-8-stimulated phasic contractions in rabbit SO.
The results showed that the CCK-8-induced increase in phasic contractile activity characterized by an amplification of frequency of contractions superimposed on an increase in tension was augmented by NO synthase inhibition. The amplitude of contractions, exhibited only a tendency to increase when the effect of CCK-8 on rings pre-exposed to LNNA was studied. Neutralization of VIP yielded an increase in tension and a reduction of contractile amplitude with no effect on frequency of contraction. NO synthase inhibition together with neutralizing VIP completely blocked any phasic activity after CCK-8 with a substantial increase in tension i.e., this combined treatment converted the phasic activity stimulating effect of CCK-8 to a pure increase in tonic contraction.
As far as the mechanism of action of CCK on the SO is concerned, previously it has been postulated that CCK at least in the dog sphincter exerts the majority of its effect through stimulation of receptors on smooth muscle cells[9]. Nevertheless, results by Behar and Biancani[10] revealed that blockade of action potential propagation by TTX abolishes the inhibitory effect of CCK on sphincter mechanics converting its action to stimulation. This evokes the concept of the involvement of neural inhibitory mechanisms in the CCK effect that interact with stimulatory impulses targeted to smooth muscle receptors. Regarding neural inhibition on SO of rabbits, we have shown that it is primarily of nitrergic origin[4]. NO besides producing smooth muscle relaxation may enhance the release of VIP, which in turn may further stimulate NO formation[7]. Moreover, CCK A receptors have been found to stimulate VIP and acetylcholine release from enteric nerve terminals at pre-and post-junctional sites, whereas CCK B receptors seem to elicit NO release at post-junctional sites[8]. The results that neither LNNA nor VIPa was able to block the phasic activity stimulating effect of CCK-8 but combined application of these substances resulted in conversion of CCK-8 effects to a full contractile one seem to support the assumption that an interplay between VIP and NO is of crucial importance in the development of phasic activity by CCK-8 in the rabbit SO. Moreover, since both TTX and atropine could block the effect of CCK-8, the majority of the effects of CCK-8 seem to be mediated by cholinergic impulses.
To the best of our knowledge this report is the first to describe that the integrity of neural nitrergic and VIP-mediated processes are pre-requisites for the ability of CCK-8 to stimulate sphincter motility. Since in rapacious animals, the peristaltic pump-like sphincter activity actively squeezes bile into the duodenum, and this sphincter activity is underlain by neurotransmitter release from either intrinsic or sensory neurons, it is not surprising that diseases that deteriorate these nerves impair sphincter function as well[11,12]. Of course, based on the present results it is not possible to estimate whether or not the neural responses participate in CCK-8-induced responses. However, that VIP and NO together comprise the principle neural effectors of relaxation interrupting tonic contractions evoked by CCK-8 in the rabbit SO seem to be supported by the data obtained. Similarly, the present work has not elucidated the precise role of cholinergic impulses implicated in either contractile or relaxant effects of CCK-8. Nevertheless, since similar to that seen with TTX, atropine also blocked the CCK-8 effects, thus it is possible that cholinergic nerves besides eliciting contractions may facilitate relaxation through releasing NO or VIP or other currently undefined relaxants.
| 1. | Toouli J, Baker RA. Innervation of the sphincter of Oddi: physiology and considerations of pharmacological intervention in biliary dyskinesia. Pharmacol Ther. 1991;49:269-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Pauletzki JG, Sharkey KA, Davison JS, Bomzon A, Shaffer EA. Involvement of L-arginine-nitric oxide pathways in neural relaxation of the sphincter of Oddi. Eur J Pharmacol. 1993;232:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Wiley JW, O'Dorisio TM, Owyang C. Vasoactive intestinal polypeptide mediates cholecystokinin-induced relaxation of the sphincter of Oddi. J Clin Invest. 1988;81:1920-1924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Lonovics J, Jakab I, Szilvássy J, Szilvássy Z. Regional differences in nitric oxide-mediated relaxation of the rabbit sphincter of Oddi. Eur J Pharmacol. 1994;255:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Slivka A, Chuttani R, Carr-Locke DL, Kobzik L, Bredt DS, Loscalzo J, Stamler JS. Inhibition of sphincter of Oddi function by the nitric oxide carrier S-nitroso-N-acetylcysteine in rabbits and humans. J Clin Invest. 1994;94:1792-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109-142. [PubMed] |
| 7. | Allescher HD, Kurjak M, Huber A, Trudrung P, Schusdziarra V. Regulation of VIP release from rat enteric nerve terminals: evidence for a stimulatory effect of NO. Am J Physiol. 1996;271:G568-G574. [PubMed] |
| 8. | Vergara P, Woskowska Z, Cipris S, Fox-Threlkeld JE, Daniel EE. Mechanisms of action of cholecystokinin in the canine gastrointestinal tract: role of vasoactive intestinal peptide and nitric oxide. J Pharmacol Exp Ther. 1996;279:306-316. [PubMed] |
| 9. | Lonovics J, Lenart Z, Velosy B, Nemeth J, Varro V, Thompson JC. Differences in the mechanism cholecystokinin, substance P, and vasoactive intestinal polypeptide induced sphincter of Oddi relaxation in dogs. Penke B, Torok A, ed. New York: Walter de Gruyter 1988; 171-174. |
| 10. | Behar J, Biancani P. Effect of cholecystokinin and the octapeptide of cholecystokinin on the feline sphincter of Oddi and gallbladder. Mechanisms of action. J Clin Invest. 1980;66:1231-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 120] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Szilvassy Z, Nagy I, Szilvassy J, Jakab I, Csati S, Lonovics J. Impaired nitrergic relaxation of the sphincter of Oddi of hyperlipidaemic rabbits. Eur J Pharmacol. 1996;301:R17-R18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Szilvassy Z, Sari R, Nemeth J, Nagy I, Csati S, Lonovics J. Improvement of nitrergic relaxation by farnesol of the sphincter of Oddi from hypercholesterolaemic rabbits. Eur J Pharmacol. 1998;353:75-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |