Published online Jun 7, 2005. doi: 10.3748/wjg.v11.i21.3260
Revised: June 1, 2004
Accepted: July 8, 2004
Published online: June 7, 2005
AIM: To investigate whether there was a relationship between the liver functions and fibrosis scores of hepatitis B patients and their TNF-α, IFN-γ, IL-4, and TGF-β1 serum levels based on the studies of liver biopsies.
METHODS: Thirty patients with chronic hepatitis B (CHB) receiving no treatment and 30 healthy individuals with negative hepatitis serology and normal values of liver biochemistry were studied. After serum samples of the patients were collected, liver needle biopsy was performed on each patient. Cytokine levels were studied by ELISA. The biopsy materials were scored based on Knodell’s histological activity index.
RESULTS: In comparison of cytokine levels between CHB patients and control group, TNF-α, IL-4, and TGF-β1 levels of the patients were higher in CHB patients than in the controls, while IFN-γ level was lower in the patients than in the controls. There were significant differences between the groups in TNF-α, IL-4, TGF-β1, and IFN-γ (P<0.005, 0.03, 0.002, 0.0001, respectively). There was a negative correlation between TGF-β1 and IL-4 and IFN-γ (P<0.05), TNF-α and the other cytokines and IFN-γ and IL-4 were not correlated (P>0.05). TGF-β1 was correlated with fibrosis (P<0.05). Liver necroinflammatory activity and fibrosis and TNF-α, IL-4, and IFN-γ were not correlated (P>0.05).
CONCLUSION: In the course of HBV infection and its chronic progress, cytokines play an important role. IL-4 and IFN-γ are effective in the chronic progression, while TGF-β1 is effective in the development of fibrosis. Serum cytokine levels may be effective tools in the estimation of chronic progression and fibrosis development.
- Citation: Akpolat N, Yahsi S, Godekmerdan A, Demirbag K, Yalniz M. Relationship between serum cytokine levels and histopathological changes of liver in patients with hepatitis B. World J Gastroenterol 2005; 11(21): 3260-3263
- URL: https://www.wjgnet.com/1007-9327/full/v11/i21/3260.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i21.3260
HBV is a common infection agent leading to various clinical patterns from subclinic infection to fulminant hepatitis. While most of the hepatitis B cases fully recover, some remain carriers and some suffer from its chronic complications such as fibrosis and/or cirrhosis[1-3]. Cytokines have been claimed to have a role in the management, chronic progress, and fibrosis development in hepatitis B infections[4,5]. This study aimed to investigate whether there was a relationship between liver functions and fibrosis scores of hepatitis B patients and their TNF-α, IFN-γ, IL-4, and TGF-β1 serum levels based on the studies of liver biopsies.
Thirty patients (24 males, 6 females; mean age, 26 years) with positive HBsAg longer than 6 mo and chronic hepatitis B (CHB) diagnosis based on their serological results comprised the study group. After their serum samples were collected, they were subjected to liver needle biopsy. Thirty healthy subjects (18 males, 12 females; mean age, 22 years) with negative hepatitis serology, normal liver biochemistry values, and without acute or chronic infections served as a control group of the study.
The serum samples of the study and control groups were studied by ELISA for TGF-β1, IFN-γ, TNF-α, and IL-4 levels with cytokine kits (Biosource, CA, USA).
Echo-guided percutaneous liver biopsy was undertaken using an 18-G needle and when enough tissues were obtained (length of the specimen >2 cm), the biopsy samples were fixed in buffered formaldehyde for 24 h and then processed by routine tissue procedures. The tissue samples were then embedded into paraffin blocks and sliced into 4-μm-thick sections.
Each biopsy was analyzed simultaneously by two pathologists (NA, SY) with two hematoxylin and eosin (HE) stained sections and periodic-acid Schiff with diastase stain for necroinflammatory activity (NIA). The Masson’s trichrome and Sweet’s reticulin stains were reviewed for fibrosis and structural change. The biopsy samples were scored for NIA (grade) and fibrosis (stage) according to the modified Knodell’s histological activity index[6].
Statistical analyses of the data were conducted with ‘SPSS for Windows (ver.10.1)’. Student’s t test was used in the comparison of the study and control groups, and Spearman-Pearson correlation test was used in the evaluations of the relation among cytokines, and between cytokines and other parameters (NIA, fibrosis). P<0.05 was considered statistically significant.
In the study group, there were 24 males (80%) and 6 females (20%) with their age ranging from 16 to 55 years (mean age: 26 years). The control group consisted of 18 males (60%) and 12 females (40%) with their age ranging from 21 to 38 years (mean age: 22 years) (Table 1).
| Patients | Control | |
| n | 30 | 30 |
| Sex | ||
| Male (%) | 24 (80) | 18 (60) |
| Female (%) | 6 (20) | 12 (40) |
| Age (yr) | ||
| Age interval | 16-55 | 21-38 |
| Median age | 26 | 22 |
In comparison of cytokine levels between patients and controls, TNF-α, IL-4, TGF-β1, and IFN-γ levels were significantly higher in the patients than in the controls (P<0.005, 0.03, 0.002, 0.0001, respectively) (Table 2).
| Patients | Control | P | |
| IFN-γ | 1.57±0.59 | 3.44±0.41 | <0.0001 |
| IL-4 | 2.51±0.67 | 1.01±3.88 | <0.030 |
| TGF-β1 | 822.8±121.24 | 419.33±37.78 | <0.002 |
| TNF-α | 17.7±4.98 | 3.1±1.21 | <0.005 |
There was a significant correlation between IL-4 and TGF-β1 (P<0.05), while TGF-β1 and IFN-γ were negatively correlated (P<0.05). TNF-α and other cytokines, and IFN-γ and IL-4 were not correlated (P>0.05).
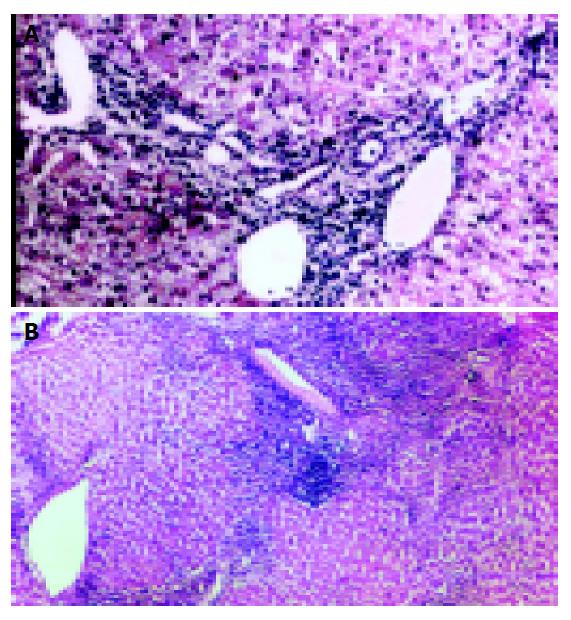
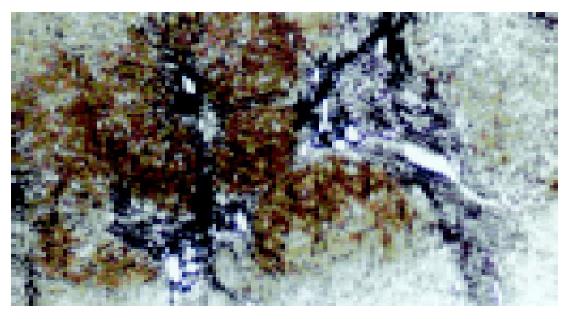
In the evaluation of the liver functions of the patients, 13 patients had minimal (Figure 1A), 7 mild, 5 moderate, and 5 severe (Figure 1B) NIA (Table 3). In the stage (fibrosis) evaluation, the results were as follows: 3 patients had no fibrosis, 11 had mild fibrosis, 14 had severe fibrosis, and 2 had cirrhosis (Figure 2). The histopathologic values of the patients are shown in Table 2.
| Fibrosis | NIA | Total | |||
| Minimal | Mild | Moderate | Severe | ||
| Absent | 3 | 0 | 0 | 0 | 3 |
| Mild | 6 | 4 | 1 | 0 | 11 |
| Severe | 4 | 3 | 4 | 3 | 14 |
| Cirrhosis | 0 | 0 | 0 | 2 | 2 |
| Total | 13 | 7 | 5 | 5 | 30 |
Fibrosis and TGF-β1 were significantly correlated (P<0.05). However, the other cytokines and fibrosis had no significant correlation (P>0.05).
Viral dissemination is mainly limited by cellular immunity. Cytotoxic T lymphocytes (Tc) play a most important role in the host defense against viruses. T-cell receptors are activated by the complex of HLA class I or II and viral proteins, during which CD4+ and CD8+ T lymphocytes play a role. Cytotoxic T lymphocytes exert their effects either by forming direct cytolysis and/or apoptosis or by cytokine secretion. In the control of immune response, CD4+ cells assume the central role in cytokine secretions, and CD8+ cells produce cytokines[5,7-9].
High levels of TNF-α, a chief mediator in many inflammatory processes, have been detected in experimental liver damages and CHB. TNF-α is known to play a key role in liver regeneration, proliferative response, and to reduce type I collagen, thus antagonizing the fibrogenic effects of TGF-β1. In vitro studies have shown that TNF-α increased HBV mRNA demolition and inhibited HBV replication[5,10].
In CHB, serum levels of TNF-α and its secretion from peripheral blood mononuclear cells were elevated in in vitro studies. In addition, by intense secretion of TNF-α from mononuclear blood cells in CHB patients under IFN-α treatment increased transaminases were detected, and elevated TNF-α levels were claimed to indicate HBV elimination[11].
In this study, TNF-α values of the patients were significantly higher than those of the controls (P<0.005), which may indicate that TNF-α can inhibit viral replication and ongoing process of HBV elimination[11].
In the management and elimination of HBV infection, increased IFN-γ secretion has been reported. IFN-γ levels were found to be lower in CHB patients than in the control group and even lower in the decompensation phase. Furthermore, a correlation between IFN-γ levels and activity was reported[7,12,13].
In comparison of serum IFN-γ (Th1 response) levels between the study and control groups, the IFN-γ (Th1 response) levels of the study group was significantly lower than those of the controls (P<0.001). However, they were not correlated with NIA (P>0.05). Lower IFN-γ levels in CHB patients than in the controls may be attributed to the reduction and/or loss of IFN-γ efficiency during chronic progression of the infection.
The number of IL-4 secreting Th cells has been reported to be higher in CHB patients than in healthy individuals, and IL-4 elevation has been attributed to the persistent HBV infection[13-15]. Similarly, in our patients, serum IL-4 (Th2 response) levels were significantly higher than those in the controls (P<0.01).
TGF-β1 (Th3 response) has an important stimulating effect on CHB and pathogenesis of fibrosis in patients with cirrhosis. Besides its immunosuppressive effect, its strong correlation with the histological degree of lobular necrosis and activity has been reported[5,7,16]. TGF-β1 may also reduce hepatocyte regeneration, enhance stellate cell activation, and trigger fibrogenesis. It could stimulate extracellular matrix proteins such as collagen and fibronectin, and activation of type I collagen and its secretion by stellate cells[17,18]. TGF-β1 level was reported to reflect the histological stage and the activation of latent TGF-β1 was reported to be the starting point of fibrogenesis[19,20].
The serum TGF-β1 levels in CHB patients in this study were significantly higher than those in the controls (P>0.01). Furthermore, TGF-β1 was significantly correlated with fibrosis (P<0.05). Thus, our findings support the findings that TGF-β1 secretion is the most important starting point of pathogenesis of fibrosis.
IL-4 and TGF-β1 levels were significantly correlated (P<0.05), while TGF-β1 and IFN-γ levels were negatively correlated (P<0.01). Lower IFN-γ levels in CHB are suggestive of chronic progression. TGF-β1 elevation, however, is suggestive of it as the only important immune mechanism in the development of fibrosis.
In conclusion, compared with the levels of healthy individuals, the TGF-β1, IL-4, and TNF-α levels in HBV patients are significantly higher and IFN-γ levels are lower. The differences are statistically significant. The positive correlation between serum TGF-β1 levels and fibrosis suggests that TGF-β1 may be a reliable marker for the development of fibrosis (cirrhosis). Evaluation of cytokine levels may contribute to the early detection of fibrosis development, thus enabling treatment at an earlier phase. The measurement of cytokine levels in blood is a convenient and non-invasive method. However, it has some disadvantages. Since the time many of the cytokines remain in blood is very short, their detection in blood is difficult, because many cytokines do not have endocrine effects, and do not pass into the blood. In HBV patients accompanying acute/chronic diseases, blood cytokine levels may not be a reliable marker.
Because of the reasons stated above, the measurement of cytokines at tissue level should prove more reliable in the estimation of the course of the disease.
| 1. | Crawford JM. The liver and the biliary tract. 6th ed. Philadelphia: WB Saunders Company 1999; 845-901. |
| 2. | Lee RG. Diagnostic Liver Pathology, 1st ed. St Luois, Mosby Company 1994: 57-79. . |
| 3. | Koff RS. Viral Hepatitis, Diseases of the liver, 7.th edition (eds. Schiff L and Shiff ER). Philadelphia, J B Lippincott Company 1993: 492-577. . |
| 4. | Gramantieri L, Casali A, Trerè D, Gaiani S, Piscaglia F, Chieco P, Cola B, Bolondi L. Imbalance of IL-1 beta and IL-1 receptor antagonist mRNA in liver tissue from hepatitis C virus (HCV)-related chronic hepatitis. Clin Exp Immunol. 1999;115:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Koziel MJ. Cytokines in viral hepatitis. Semin Liver Dis. 1999;19:157-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 164] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1582] [Cited by in RCA: 1517] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 7. | Ben-Ari Z, Mor E, Papo O, Kfir B, Sulkes J, Tambur AR, Tur-Kaspa R, Klein T. Cytokine gene polymorphisms in patients infected with hepatitis B virus. Am J Gastroenterol. 2003;98:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 180] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Jung MC, Pape GR. Immunology of hepatitis B infection. Lancet Infect Dis. 2002;2:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 196] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 9. | Guidotti LG. The role of cytotoxic T cells and cytokines in the control of hepatitis B virus infection. Vaccine. 2002;20 Suppl 4:A80-A82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Zeuzem S, Carreño V. Interleukin-12 in the treatment of chronic hepatitis B and C. Antiviral Res. 2001;52:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Bradham CA, Plümpe J, Manns MP, Brenner DA, Trautwein C. Mechanisms of hepatic toxicity. I. TNF-induced liver injury. Am J Physiol. 1998;275:G387-G392. [PubMed] |
| 12. | Schlaak JF, Tully G, Löhr HF, Gerken G, Meyer zum Büschenfelde KH. HBV-specific immune defect in chronic hepatitis B (CHB) is correlated with a dysregulation of pro- and anti-inflammatory cytokines. Clin Exp Immunol. 1999;115:508-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Jiang R, Feng X, Guo Y, Lu Q, Hou J, Luo K, Fu N. T helper cells in patients with chronic hepatitis B virus infection. Chin Med J (Engl). 2002;115:422-424. [PubMed] |
| 14. | Kobayashi K, Ishii M, Igarashi T, Satoh T, Miyazaki Y, Yajima Y, Ukai K, Suzuki H, Kanno A, Ueno Y. Profiles of cytokines produced by CD4-positive T lymphocytes stimulated by anti-CD3 antibody in patients with chronic hepatitis C. J Gastroenterol. 1998;33:500-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Atsukawa K, Saito H, Tsukada N, Akiba Y, Toda K, Kumagai N, Ohishi T, Kamegaya Y, Ishii H. Th1 and Th2 cytokines differentially regulate the transformation of Kupffer cells into multinucleated giant cells but similarly enhance the Kupffer cell-induced hepatic stellate cell proliferation. Hepatol Res. 2001;20:193-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Hirohashi S, Ishak KG, Kojiro M, Wangless IR, Theise ND, Tsukuma H. Hepatocellular Carcinoma. Hamilton SR, Aaltonen LA. Pathology and Genetics Tumours of the Digestive System. First edition. Lyon: IARC 2000; 159-172. |
| 17. | Schürch W, Gurley AM, Roth SI. Myofibroblast. Sternberg SS. Histology for Pathologists. Second edition. Philadelphia: Lippincott Raven 1997; 129-166. |
| 18. | Kweon YO, Goodman ZD, Dienstag JL, Schiff ER, Brown NA, Burchardt E, Schoonhoven R, Brenner DA, Fried MW. Decreasing fibrogenesis: an immunohistochemical study of paired liver biopsies following lamivudine therapy for chronic hepatitis B. J Hepatol. 2001;35:749-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 132] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Neuman MG, Benhamou JP, Bourliere M, Ibrahim A, Malkiewicz I, Asselah T, Martinot-Peignoux M, Shear NH, Katz GG, Akremi R. Serum tumour necrosis factor-alpha and transforming growth factor-beta levels in chronic hepatitis C patients are immunomodulated by therapy. Cytokine. 2002;17:108-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Mann DA, Smart DE. Transcriptional regulation of hepatic stellate cell activation. Gut. 2002;50:891-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 156] [Article Influence: 6.5] [Reference Citation Analysis (0)] |