Published online May 28, 2005. doi: 10.3748/wjg.v11.i20.3075
Revised: July 21, 2004
Accepted: August 25, 2004
Published online: May 28, 2005
AIM: To investigate the uptake difference between bovine serum albumin nanoparticle (BSA-NP) and bovine serum albumin nanoparticles with their surface modified by glycyrrhizin (BSA-NP-GL) and to develop a novel hepatocyte targeting BSA-NP-GL based on active targeting technology mediated by specific binding site of GL on rat cellular membrane.
METHODS: Calcein loaded bovine serum albumin nanoparticles (Cal-BSA-NP) were prepared by desolvation process. Glycyrrhizin was conjugated to the surface reactive amino groups (SRAG) of Cal-BSA-NP by sodium periodate oxidization, which resulted in calcein-loaded bovine serum albumin nanoparticles with their surface modified by glycyrrhizin (Cal-BSA-NP-GL). The morphology of the two types of prepared nanoparticles (NP) was observed by transmission electron microscopy. The diameter of NP was measured with a laser particle size analyzer. The interaction between Cal-BSA-NP-GL and primary cultured hepatocytes was studied through cellular uptake experiments. The uptake amount of Cal-BSA-NP-GL and Cal-BSA-NP by rat hepatocytes was determined by fluorospectrophotometry. Uptake characteristics were investigated through experiments of competitive inhibition of specific binding site of GL.
RESULTS: Both Cal-BSA-NP-GL and Cal-BSA-NP had regular spherical surfaces. The average diameter of Cal-BSA-NP-GL and Cal-BSA-NP was 77 and 79 nm respectively. The uptake amount of the two NP by hepatocytes reached its maximum at 2 h after incubation. The uptake amount of Cal-BSA-NP-GL by rat hepatocytes was 4.43-fold higher than that of Cal-BSA-NP. There was a significant difference in the uptake of Cal-BSA-NP-GL and Cal-BSA-NP by hepatocytes (P<0.01). The uptake of Cal-BSA-NP-GL was inhibited when GL was added previously to isolated rat hepatocytes, and the uptake of Cal-BSA-NP was not affected by GL.
CONCLUSION: A binding site of GL is present on the surface of rat hepatocytes, BSA-NP-GL may be internalized via this site by hepatocytes and can be used as a drug carrier for active targeting of delivery drugs to hepatocytes.
- Citation: Mao SJ, Hou SX, He R, Zhang LK, Wei DP, Bi YQ, Jin H. Uptake of albumin nanoparticle surface modified with glycyrrhizin by primary cultured rat hepatocytes. World J Gastroenterol 2005; 11(20): 3075-3079
- URL: https://www.wjgnet.com/1007-9327/full/v11/i20/3075.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i20.3075
Liver cells are divided into hepatocytes (hepatic parenchymal cells), Kupffer cells and endothelial cells (hepatic nonparenchymal cells). Parenchymal cells are the main cells of liver. Many fatal diseases occur in hepatocytes, such as chronic hepatitis, cirrhosis, enzyme deficiency and hepatoma. Because of the poor efficiency of current drugs uptaken by hepatocytes, there are few effective therapeutic methods for hepatic diseases. Hence, it is important to target drugs to the hepatocytes.
Application of nanoparticles (NP) in drug delivery systems (DDS) is of particular interest because they provide drug targeting possibilities and sustained release action. However, they have some disadvantages such as rapid uptake by the reticuloendothelial system (RES) within seconds or minutes after injection due to phagocytosis by macrophages in the liver and spleen.
Receptor-mediated drug targeting is a promising approach to selective drug delivery. One particular method exploits the mechanisms of sugar recognition of some specific cells. Among various types of cells in the body, hepatocytes exclusively have high affinity cell-surface receptors that can bind to asialoglycoproteins and subsequently internalize them to the cell interior. Delivery of drugs using liposomes bound to asialoglycoprotein in a specific manner would provide significant therapeutic benefits to hepatic disease. Extensive studies on chemical modification of liposomes with asialoglycoproteins[1-3] or low-molecular weight glycolipid have been carried out to achieve effective targeting to hepatocytes[4-8]. However, it was reported that galactose-bearing liposomes are also taken up by Kupffer cells, and the distribution of these liposomes in hepatocytes is not very high[9]. Therefore, the discovery of a new ligand instead of using the conventional ones requires investigation.
Glycyrrhizin (GL), a conjugate of one molecule of glycyrrhetinic acid and two molecules of glucuronic acid, is one of the main compounds extracted from the root of Glycyrrhiza glabra L (licorice). In 1990s, Negishi et al[10], proved that there are specific binding site of glycyrrhizin on the cellular membrane of in vitro rat hepatocytes. Based on this research, liposome surface modified with glycyrrhizin was prepared and proved that the uptake amount and in vivo distribution of the modified liposomes are considerably higher compared with the conventional liposomes[11,12].
However, liposomes have limitations due to several factors such as leakage of their contents before reaching the target tissue, rapid clearance from the blood stream and their uptake by macrophages of the liver and spleen (RES). Therefore, it is necessary to develop a novel hepatocyte targeting DDS. We have previously prepared albumin NP with their surface modified by glycyrrhizin and studied their pharmaceutical characteristics[13]. In this study, calcein-loaded bovine serum albumin nanoparticles with their surface modified by glycyrrhizin (Cal-BSA-NP-GL) was prepared and the uptake of Cal-BSA-NP-GL by in vitro rat hepatocytes was investigated. The interaction between Cal-BSA-NP-GL and isolated rat hepatocytes was determined and the uptake mechanism and influential factors were initially investigated through in vitro cell culture experiments.
Bovine serum albumin was purchased from Bio Life Science & Technology Co. Ltd (Shanghai, China). Calcein was obtained from SSS Reagent (Shanghai, China). Glycyrrhizin, desoxyribonuclease I and collagenase IV were purchased from Sigma (USA). Carbonate buffer (CBS, pH 9.50, 0.2 mol/L Na2CO3 13.0 mL+0.2 mol/L NaHCO3 37.0 mL), Hank’s solution and RPMI-1640 culture medium were provided by the Department of Immunology, School of Preclinical Medicine, Sichuan University. All the other chemicals and reagents used were of analytical grade.
Albumin NP were prepared by a desolvation process as described previously by Weber et al[14]. In brief, 100 mg bovine serum albumin was dissolved in 10 mL solvent of calcein (0.1 mg/mL) by constant stirring at 25 °C, 20 mL ethanol and 25 μL 2.5% glutaral solution were added gradually into the solvent mixture respectively. After 3-h stirring, ethanol was vacuum distilled and the colloidal solution of calcein-loaded bovine serum albumin nanoparticles (Cal-BSA-NP) was obtained. Glycyrrhizin was dissolved in CBS to make glycyrrhizin solution (0.1 mol/L), 0.1 mol/L NaIO4 solution was added gradually into the glycyrrhizin solution, which resulted in glycyrrhizin oxide solution after stirring for 3 h. Twenty milliliters of glycyrrhizin oxide solution was added into the Cal-BSA-NP solution. After being stirred for 3 h at 25 °C, Cal-BSA-NP-GL was obtained. The drug amount loaded on NP was determined by fluorospectrophotometry (RF-5000, Shimadzu, Japan), the particle shape was observed under a transmission electron microscope (H-600, Hitachi, Japan) and the particle size was measured with a laser particle size analyzer (Malvern-2000, UK).
Hepatocytes were isolated from normal liver of male Wistar rats weighing approximately 200 g. The livers were purged with Hank’s solution and scissored into tissue blocks (1 mm×1 mm). The blocks were treated with 1 mg/mL collagenase IV solution (pH was adjusted to 7.4 using Hepes) at 37 °C for 120 min and filtrated via a 200-mesh cell sieve. Hepatocytes were washed thrice by slow centrifugation (500 r/min, 1 min) of the cell suspension to remove cell debris, damaged and nonparenchymal cells. Viability as tested by trypan blue exclusion was over 95%. Isolated hepatocytes were plated onto collagen-coated six-well culture plates at a density of 1×106 cells/well, incubated in RPMI-1640 solution in 50 mL/L CO2 at 37 °C for 24 h, and used in the following experiments.
Cal-BSA-NP and Cal-BSA-NP-GL solutions were ultracentrifuged (1.5×104g) for 20 min, the supernatants were discarded and precipitations were ultrasonically dispersed with 0.5 mL PBS. Then, 0.5 mL Cal-BSA-NP and 0.5 mL Cal-BSA-NP-GL solutions were added to 1 mL preincubated hepatocyte suspension respectively, RPMI-1640 culture solution was added to 2 mL and mixed uniformly. Hepatocytes were incubated with Cal-BSA-NP and Cal-BSA-NP-GL at the indicated concentration in a fresh medium. After incubation for the indicated time, the medium was removed and the cells were washed four times with 12 mL ice-cold PBS, 2 mL distilled water was added into the washed cells and the mixture was stored at -10 °C for 2 h. The congelations were defrosted at room temperature to disintegrate the cells. The achieved mixture was centrifuged for 10 min (1×104g), and the supernatants were diluted with PBS to 5 mL. The fluorescence strength in the solutions was determined (λex = 491.2 nm, λem = 510.4 nm, slit width = 10 nm). When the concentration of calcein was 0.2-6.4 ng/mL, the standard curve was described as regression equation: F = -1.51+97.58C (ng/mL), r = 0.9997. The uptake amount of the two types of NP by hepatocytes was expressed as nanograms of total BSA per 106 cells. Each value represented the mean±SD of three experiments. The data were calculated according to the drug amount loaded and the determined strength of fluorescence. For the inhibition experiment, the cells were preincubated with GL solution (GL dissolved in 50 mg/mL NaHCO3) at a concentration of 0-50 mmol/L in medium. The uptake experiments were then carried out as above.
In order to exclude the error caused by calcein absorption of the cell culture plate, parallel processes were followed in all experiments. 0.5 mL Cal-BSA-NP and Cal-BSA-NP-GL solutions were incubated, centrifuged, purged and treated with the above-mentioned method, their strength of fluorescence was determined and deducted while the uptake quantity of hepatocytes was calculated.
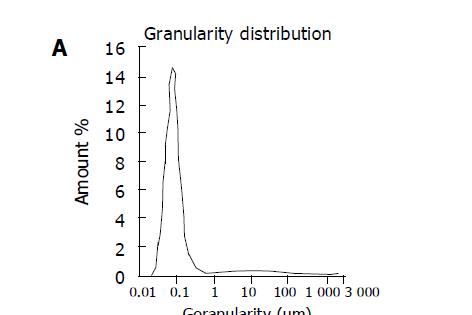
The drug amount loaded on Cal-BSA-NP and Cal-BSA-NP-GL was 6.02 and 5.93 µg/mg BSA respectively. Transmission electron microscopy (Figure 1) demonstrated that both Cal-BSA-NP (Figure 1A) and Cal-BSA-NP-GL (Figure 1B) had a regular spherical surface. Figure 2 showed that the average diameter of Cal-BSA-NP and Cal-BSA-NP-GL was 77 and 79 nm respectively with a narrow distribution (polyindex 0.42, 0.31).
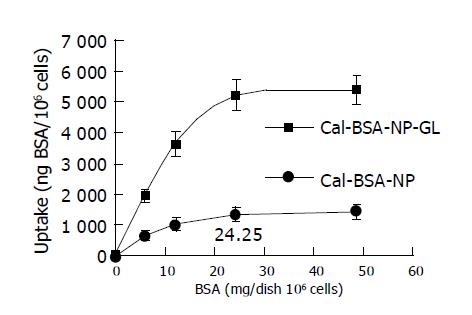
In these experiments, 0.5 mL Cal-BSA-NP and Cal-BSA-NP-GL solutions at the indicated concentration were added respectively to 1 mL previously incubated hepatocyte suspensions, and 0.5 mL RPMI-1640 culture solution was added to each mixture to 2 mL. The amount of BSA added to each culture dish was 6.08, 12.16, 24.25, and 48.58 mg. The mixtures were incubated in 50 mL/L CO2 at 37 °C for 2 h. The dose dependency of the uptake of Cal-BSA-NP and Cal-BSA-NP-GL by primary cultured rat hepatocytes was examined, and the results are shown in Figure 3. Cal-BSA-NP-GL was taken up by rat hepatocytes to a much greater extent than the control Cal-BSA-NP. The uptake of Cal-BSA-NP-GL increased dose-dependently and was saturated at 5220 ng BSA/dish (106 cells) at a dose of 24.25 mg BSA/dish. However, the uptake of Cal-BSA-NP was slightly and linearly increased.
The time course of the uptake of Cal-BSA-NP and Cal-BSA-NP-GL by hepatocytes is shown in Figure 4. In these experiments, a high affinity of Cal-BSA-NP-GL for hepatocytes was observed. The concentration of calcein in each culture well was 29.1 μg/mL (corresponding BSA in each culture dish was 24.25 mg), at which the uptake of hepatocytes was saturated. The mixtures were incubated in 50 mL/L CO2 at 37 °C for 0, 1, 2, 3, and 4 h respectively, and the incubation was stopped at the indicated time. The incubated mixtures were dealt with following the process in the section “In vitro hepatocytes uptake experiment”. The time course of the uptake of Cal-BSA-NP-GL was obtained as a biphase curve. The uptake of Cal-BSA-NP-GL and Cal-BSA-NP by hepatocytes reached its maximum after the NP were incubated for 2 h. The uptake amount of Cal-BSA-NP-GL by rat hepatocytes was 4.43-fold higher than that of Cal-BSA-NP. There was a significant difference between Cal-BSA-NP-GL and Cal-BSA-NP (P<0.01).
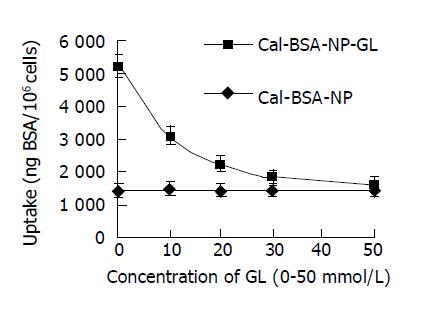
The high affinity of Cal-BSA-NP-GL for hepatocytes is thought to be due to GL. The presumptive sites on hepatocytes to which Cal-BSA-NP-GL bind are assumed to be specific ones for GL. To demonstrate this theory, we examined whether the uptake of Cal-BSA-NP-GL was inhibited by free GL. Glycyrrhizin was dissolved and diluted to 0.1 mol/L with 50 mg/mL NaHCO3, and 0, 0.2, 0.4, 0.6, and 1.0 mL of glycyrrhizin solution were added to preincubated hepatocyte suspensions and the mixtures were incubated in 50 mL/L CO2 at 37 °C for 5 min. Then, 0.5 mL Cal-BSA-NP-GL and Cal-BSA-NP solutions were added to the mixtures respectively, and RPMI-1640 culture solution was added to each mixture to 2 mL (the amount of BSA in each culture dish was 24.25 mg). The culture plate was incubated in 50 mL/L CO2 at 37 °C for 2 h. An identical process was carried out to measure the strength of fluorescence of cell precipitation and the result is shown in Figure 5. The uptake of Cal-BSA-NP-GL was inhibited in response to the concentration of GL in the medium, and the inhibitory ratio was 73% at the concentration of 50 mmol/L of GL.
In order to modify the BSA-NP surface with GL, GL was converted into a glycyrrhizin oxide. Aldehyde group of obtained glycyrrhizin oxide was conjugated with surface reactive amino groups of BSA-NP to form Schiff bases, a novel hepatocytes targeting DDS was successfully prepared.
In in vitro hepatocyte uptake experiments, the uptake of modified NP depended on the dosage. However, the uptake of Cal-BSA-NP slightly increased linearly. The uptake of drug-loaded NP with their surface modified with glycyrrhizin by hepatocytes could be saturated when the dosage was high enough. Saturation appeared in the dose-response curve of the uptake of Cal-BSA-NP-GL, indicating that the specific binding site of glycyrrhizin is limited on the surface of hepatocytes. The time course of the uptake of NP by hepatocytes accords with the rule of biphase dynamics. During the initial 1 h of incubation, Cal-BSA-NP-GL was rapidly taken up by the cells, and subsequently the uptake of Cal-BSA-NP-GL became slow and increased proportionally to the incubation time. When exogenous glycyrrhizin was added, the uptake of drug-loaded NP with their surface modified with glycyrrhizin, by hepatocytes decreased with increase in the concentration of glycyrrhizin, and the uptake amount approached to traditional NP at last. At the same circumstances, the uptake of traditional NP by hepatocytes increased slowly and linearly. No inhibitory effect was shown on the uptake of Cal-BSA-NP after the addition of GL, suggesting that GL does not diminish the ability of hepatocytes to take up BSA-NP. If the presence of a binding site specific for GL on the surface of hepatocytes is considered, Cal-BSA-NP-GL is likely to bind to this site with the GL moiety. If the specific binding site of glycyrrhizin on the surface of hepatocytes is saturated by exogenous glycyrrhizin, the internalization of Cal-BSA-NP-GL mediated by specific binding site of glycyrrhizin is decreased, with the addition of glycyrrhizin, which is a rational explanation of the excessive uptake of Cal-BSA-NP-GL by hepatocytes compared with Cal-BSA-NP.
It was reported that the uptake of liposomes with their surface modified with glycyrrhizin by hepatocytes increases 10-fold as many as that of traditional liposomes[11]. In our research, the uptake of Cal-BSA-NP-GL by hepatocytes increased 4.43-fold as many as that of Cal-BSA-NP. It might be caused by the following factors. The surface of albumin NP is hydrophilic, its affinity to cell membrane is weaker than liposomes. The structure of bimolecular phospholipid layer of liposomes is similar to that of hepatocyte membrane, the flexible affluxion of phospholipid layer of liposomes makes it easier for GL to combine with the specific binding site of glycyrrhizin on the surface of hepatocyte membrane. The amount of glycyrrhizin on the surface of NP with their surface modified by glycyrrhizin is not enough. The results indicate that the amount of specific binding site of glycyrrhizin on the surface of hepatocyte membrane cannot determine the specific uptake of NP with their surface modified by glycyrrhizin. The ability of GL to combine with the specific binding site of glycyrrhizin and the characteristics of the surface of NP also play an important role in the specific uptake of NP with their surface modified by glycyrrhizin by hepatocytes.
In conclusion, the binding site of GL are present on the surface of rat hepatocytes, BSA-NP-GL may be internalized via this site by hepatocytes and can be used as a promising drug carrier for active targeting of delivery drugs to hepatocytes.
| 1. | Tsuchiya S, Aramaki Y, Hara T, Hosoi K, Okada A. Preparation and disposition of asialofetuin-labelled liposome. Biopharm Drug Dispos. 1986;7:549-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Ishihara H, Hara T, Aramaki Y, Tsuchiya S, Hosoi K. Preparation of asialofetuin-labeled liposomes with encapsulated human interferon-gamma and their uptake by isolated rat hepatocytes. Pharm Res. 1990;7:542-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Wu J, Liu P, Zhu JL, Maddukuri S, Zern MA. Increased liver uptake of liposomes and improved targeting efficacy by labeling with asialofetuin in rodents. Hepatology. 1998;27:772-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Spanjer HH, Scherphof GL. Targeting of lactosylceramide-containing liposomes to hepatocytes in vivo. Biochim Biophys Acta. 1983;734:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 88] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Ghosh PC, Bachhawat BK. Targeting of liposomes to hepatocytes. Targeted Diagn Ther. 1991;4:87-103. [PubMed] |
| 6. | Kawakami S, Yamashita F, Nishikawa M, Takakura Y, Hashida M. Asialoglycoprotein receptor-mediated gene transfer using novel galactosylated cationic liposomes. Biochem Biophys Res Commun. 1998;252:78-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 119] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Kawakami S, Fumoto S, Nishikawa M, Yamashita F, Hashida M. In vivo gene delivery to the liver using novel galactosylated cationic liposomes. Pharm Res. 2000;17:306-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 127] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Hirabayashi H, Nishikawa M, Takakura Y, Hashida M. Development and pharmacokinetics of galactosylated poly-L-glutamic acid as a biodegradable carrier for liver-specific drug delivery. Pharm Res. 1996;13:880-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Spanjer HH, Morselt H, Scherphof GL. Lactosylceramide-induced stimulation of liposome uptake by Kupffer cells in vivo. Biochim Biophys Acta. 1984;774:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Negishi M, Irie A, Nagata N, Ichikawa A. Specific binding of glycyrrhetinic acid to the rat liver membrane. Biochim Biophys Acta. 1991;1066:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 130] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Osaka S, Tsuji H, Kiwada H. Uptake of liposomes surface-modified with glycyrrhizin by primary cultured rat hepatocytes. Biol Pharm Bull. 1994;17:940-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Tsuji H, Osaka S, Kiwada H. Targeting of liposomes surface-modified with glycyrrhizin to the liver. I. Preparation and biological disposition. Chem Pharm Bull (Tokyo). 1991;39:1004-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Mao SJ, Hou SX, Zhang LK, Jin H, Bi YQ, Jiang B. Preparation of bovine serum albumin nanoparticles surface-modified with glycyrrhizin. YaoXue XueBao. 2003;38:787-790. [PubMed] |
| 14. | Weber C, Coester C, Kreuter J, Langer K. Desolvation process and surface characterisation of protein nanoparticles. Int J Pharm. 2000;194:91-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 396] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
Science Editor Wang XL Language Editor Elsevier HK