Published online May 28, 2005. doi: 10.3748/wjg.v11.i20.3046
Revised: June 30, 2004
Accepted: July 22, 2004
Published online: May 28, 2005
AIM: To explore the relationship between matrix metallopr-oteinase-2 (MMP-2) and tissue inhibitor of metallopr-oteinase-2 (TIMP-2) in the development of colorectal carcinoma and to provide a valuable marker for clinical diagnosis.
METHODS: Twenty-five patients with colorectal carcinoma underwent surgical resection. Samples were taken from tumor sites and normal tissues. MMP-2 activity was determined by gelatin zymography. Western blot and ABC immunohist-ochemical staining were used to detect the expression levels of MMP-2 and TIMP-2 in normal and colorectal carcinoma tissues. Statistical analyses were performed using the Student’s t test and one-way ANOVA. P<0.05 was considered statistically significant. All the statistical analyses were performed using SPSS 10.0 software.
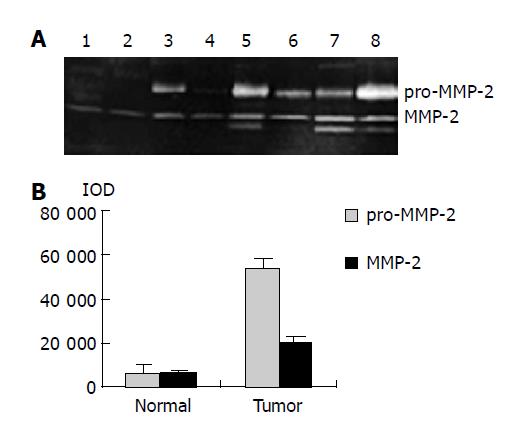
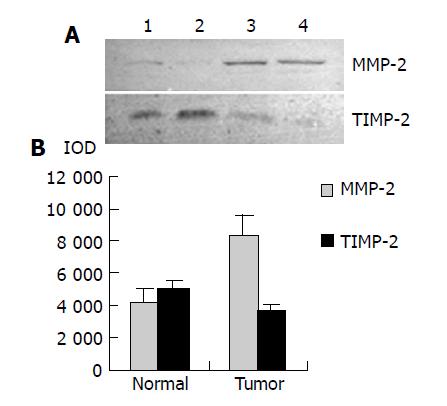
RESULTS: MMP-2 activity could be detected in both normal and colorectal carcinoma tissues. MMP-2 activity in colorectal carcinoma tissues was much higher than that in normal tissues (P<0.05, t = 3.916, 4.227). MMP-2 activity was positively related to the colorectal carcinoma invasion depth, lymph node metastasis and Duke’s stage. Western blot and ABC immunohistochemical staining demonstrated that the expression level of MMP-2 in colorectal carcinoma tissues was much higher than that in normal tissues (P<0.05, t = 9.429), but the expression level of TIMP-2 in colorectal carcinoma tissues was much lower than that in normal tissues (P<0.05, t = 7.329). The MMP-2/TIMP-2 ratio of colorectal carcinoma was much higher than that of normal tissues. With the progression of invasion depth, lymph node metastasis and tumor Duke’s stage, the activity and expression level of MMP-2 and TIMP-2 gradually increased, but the MMP-2/TIMP-2 ratio gradually decreased.
CONCLUSION: The balance between MMP-2 and TIMP-2 plays a crucial role in the process of colorectal carcinoma invasion and metastasis.
- Citation: Li BH, Zhao P, Liu SZ, Yu YM, Han M, Wen JK. Matrix metalloproteinase-2 and tissue inhibitor of metallo-proteinase-2 in colorectal carcinoma invasion and metastasis. World J Gastroenterol 2005; 11(20): 3046-3050
- URL: https://www.wjgnet.com/1007-9327/full/v11/i20/3046.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i20.3046
Colorectal carcinoma is one of the most common malignant tumors with a relatively high incidence in China. Despite major advances in the diagnosis and treatment of this disease, its mortality has remained unchanged during the last 20 years. Tumor invasion and metastasis are considered to be the major causes of death in colorectal carcinoma patients. Recent researches in the field of mechanism for tumor invasion and metastasis have demonstrated that the degradation of extracellular matrix (ECM) and basement membrane (BM) is an essential step and the contribution of matrix metallopro-teinases (MMPs) is very important during this process[1-4]. MMPs are a family of zinc-dependent endope-ptidases that are collectively capable of degrading most components of the BM and ECM[5]. Several studies have proved that there are high expression level and activity of MMPs in many kinds of tumors, such as carcinoma of esophagus, lung, stomach, etc. Tissue inhibitors of metalloproteinases (TIMPs), as the main endogenous inhibitors of the metalloproteinases, can reversibly inhibit MMPs in a 1:1 stoichiometric fashion and influence the process of tumor invasion and metastasis[6]. At present, there have been many reports about the overex-pression of matrix metalloproteinase-2 (MMP-2) in colorectal carcinoma tissues. However, few reports concerning the detailed pathophysiological significance of MMP-2 and the relationship between MMP-2 and tissue inhibitor of metalloproteinase-2 (TIMP-2) are available. Therefore, the aim of the present study was to investigate the expression characteristics of MMP-2 and TIMP-2 in colorectal carcinoma tissues and to explore the relationship between MMP-2 and TIMP-2 and the correlation with colorectal carcinoma progression, trying to provide a valuable marker for its clinical prognosis.
Twenty-five patients with colorectal carcinoma were recruited at the Fourth Hospital of Hebei Medical University from March 2002 to July 2002. Paired colorectal tumor and normal mucosal tissue samples (taken at a site 10 cm or more from the primary tumor) were collected immediately after surgical resection. Of the 25 cases, 9 had colon carci-noma, 16 had rectal carcinoma. All colorectal tumors were confirmed by their pathological examination. All the specimens were divided into two parts. One part was stored at -70 °C, the other part was fixed quickly into formalin solution at pH 7.0, embedded in paraffin and cut into sections (4-μm thick).
MMP-2 activity was analyzed by gelatin zymography. Frozen samples were homogenized in lysis buffer containing 100 mmol/L Tris-Cl, pH 7.6, 20 mmol/L NaCl, 1% Triton X-100. The lysates were incubated on ice for 30 min and insoluble materials were removed by centrifugation. Samples containing 100 μg of protein were mixed with 5×sample (4:1) and electrophoresed (120 V) for 2-3 h at 4 °C. When the tracking dye at the front reached the bottom of the gel, the gel was removed and shaken gently for 30 min in 2.5% Triton X-100 to remove SDS. Then, the gel was incubated in 50 mmol/L Tris-HCl, pH 8.0, 50 mmol/L NaCl, 10 mmol/L CaCl2, 1% Triton X-100 for 9 h at 37 °C. At last, following staining with 0.5% Coomassie brilliant blue R250 for 1.5-2 h and decolorized for 1 h. The clear band against a blue background representing the activity of MMP-2 was measured by using a gel image system (Image master 1D analysis software).
MMP-2 and TIMP-2 protein levels in normal and colorectal carcinoma tissues were determined by Western blot analysis. Tissue homogenates were prepared as described above for gelatin zymography. Five times loading buffer was added to an equal amount (150 μg) of protein extracts, which was subsequently boiled for 5 min. Then proteins were subjected to SDS-PAGE and transferred to the PVDF membrane for immunodetection. The PVDF membranes were blocked in 5% nonfat milk for 3 h at room temperature and then probed with anti-MMP-2 or anti-TIMP-2 antibody for 9 h at 4 °C. After that, the membranes were washed thrice with TBS-Tween and incubated with secondary antibody for 2 h at room temperature. Immunoreactive proteins were detected using a color development system (NBT/BCIP) by the standard procedure.
Streptavidin–biotin complex (SABC) method was adopted in formalin-fixed, paraffin-embedded sections. Sections were deparaffinized and incubated with 1% hydrogen peroxide in methanol for 10 min to block the endogenous peroxidase activity. After being washed with phosphate-buffered saline (PBS), sections were treated with 0.01 mol/L citric acid buffer to recover the antigen activity. After that, the sections were incubated with 5% normal goat serum for 30 min at room temperature and then incubated with primary antibodies to MMP-2 and TIMP-2 at an antibody concentration of 1:200, overnight at 4 °C. The negative control was used with PBS buffer replacing the polyclone antibody. ABC immunohistochemical kits and DAB substrate solution were used to detect the immune complex. The procedures of blocking, linkage, and labeling of binding reaction were carried out according to manufacturer’s instructions. The peroxidase activity was visualized by a DAB kit (Sino-American Biotechnology Co.). The deep yellow particles were defined as positive.
All the experiments were repeated thrice. Statistical analyses were performed using the Student’s t test and one-way ANOVA. P<0.05 was considered statistically significant. All the statistical analyses were performed using SPSS 10.0 software.
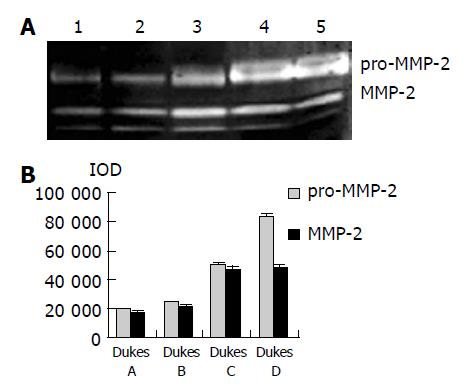
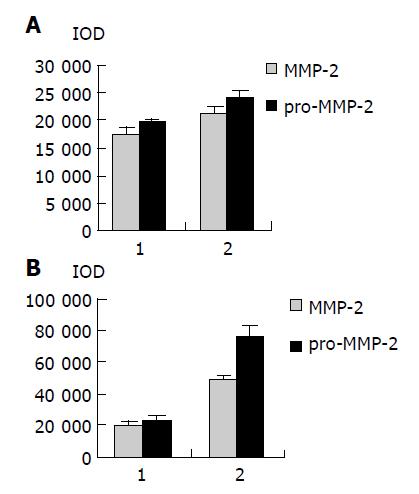
As shown in Figure 1, the major band was 72000 Mr and the minor band was 62000 Mr on SDS-PAGE. They corresponded to the latent and active forms of MMP-2 respectively. The MMP-2 activity was much higher in colorectal carcinoma tissues than in normal tissues (Figure 1, P<0.05, t = 3.916, 4.227). Further, the invasive and metastatic capacity of colorectal carcinoma was significantly correlated with the MMP-2 activity. With the progression of tumor Duke’s stage, the MMP-2 activity gradually increased (Figure 2, P<0.05, F = 219.296). As shown in Figure 3, the MMP-2 activity was much higher in the lymph node metastatic group than in the group without lymph node metastasis (Figure 3, P<0.05, t = 6.042, 20.174). The MMP-2 activity was also higher in colorectal carcinoma tissues with subserosal invasion than in those with muscularis propria invasion (Figure 3, P<0.05, t = 4.341, 3.151).
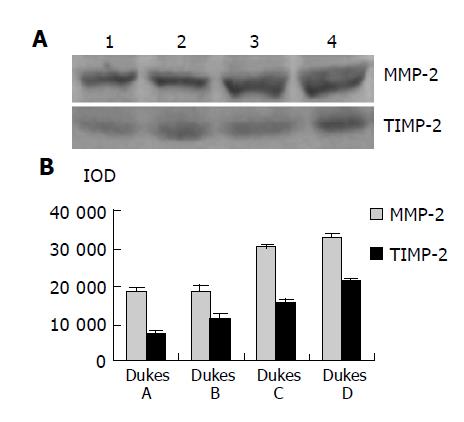
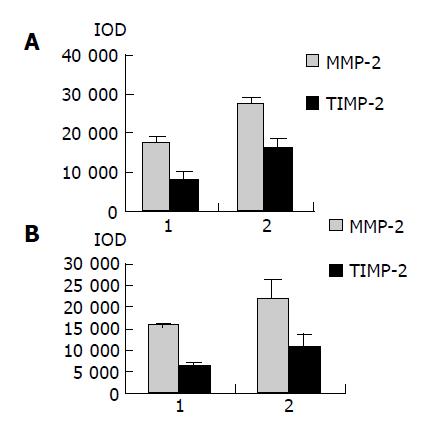
As shown in Figure 4, Western blot analysis revealed the specific bands of MMP-2 (72000 Mr) and TIMP-2 (22000 Mr) in normal and colorectal carcinoma tissues. The MMP-2 expression level was found to be significantly increased whereas significantly decreased in colorectal carcinoma tissues in comparison with normal colorectal tissues (P<0.05, t = 9.429, 7.329). The MMP-2/TIMP-2 ratio of colorectal carcinoma was much higher than that of normal tissue (Table 1, P<0.05, t = 6.474). With the progression of tumor Duke’s stage, the MMP-2 and TIMP-2 expression levels were gradually increased (Figure 5, P<0.05, F = 74.318, 124.426). The ratio of MMP-2/TIMP-2 was gradually decreased (Table 1, P<0.05, F = 6.330). It also could be seen that the MMP-2 and TIMP-2 expression levels in colorectal carcinoma tissues with regional lymph node metastasis were significantly high, while they were very low in those without lymph node metastasis (Figure 6, P<0.05, t = 10.361, 5.609). The ratio of MMP-2/TIMP-2 in the former was lower than the latter (Table 1, P>0.05, t = 2.252). The MMP-2 and TIMP-2 expression levels were much higher in colorectal carcinoma tissues with subserosal invasion than in those with muscularis propria invasion (Figure 6, P<0.05, t = 3.042, 4.004), the MMP-2/TIMP-2 ratio in the former was also lower than that in the later (Table 1, P>0.05, t = 2.476).
| Variable | MMP-2/TIMP-2 | P |
| Tissues | P<0.05 | |
| Normal | 0.5807±0.2061 | |
| Carcinoma | 2.4179±0.6534 | |
| Invasion depth | ||
| Muscular layer invasion | 2.5781±0.4336 | |
| Serous membrane layer or | ||
| surrounding soft tissue invasion | 2.1308±0.7311 | |
| Lymph node metastasis | ||
| Negative | 2.2907±0.5102 | |
| Positive | 1.7643±0.2597 | |
| Duke’s stage | ||
| A | 2.6419±0.5429 | |
| B | 1.7081±0.3631 | |
| C | 1.9880±0.1551 | |
| D | 1.5407±0.1794 |
Figure 7 shows the expression and localization of MMP-2 and TIMP-2 in normal and colorectal carcinoma tissues. Immunohistochemical staining for MMP-2 revealed that normal colorectal tissues were slightly positive while colorectal carcinoma tissues in early stage were strongly positive. In contrast, the positive staining with TIMP-2 in normal colorectal carcinoma tissues was much higher than that in colorectal carcinoma tissues. MMP-2 immunoreactivity was observed mainly in the cytoplasm of colorectal carcinoma cells and matrix around tumor cells. It was also observed frequently in stromal cells. However, the positive staining with TIMP-2 was mainly distributed in the matrix around the tumor.
Our results in this study demonstrated that MMP-2 activity and expression level were much higher in colorectal carcinoma tissues than in normal tissues. Moreover, there was a significant positive correlation between the MMP-2 activity or expression level and tumor invasion depth, lymphatic metastasis and tumor Duke’s stage. These results strongly indicated that MMP-2 expression level was not only associated with the development of colorectal carcinoma, but also played a very important role in the process of colorectal carcinoma invasion and metastasis. Immunohistochemical staining for MMP-2 identified that MMP-2 positive staining mainly occurred in the cytoplasm of colorectal carcinoma cells and matrix around them. It also frequently occurred in stromal cells. This suggests that the major cell source of MMP-2 in colorectal carcinoma is heterogeneous. Western blot analysis also demonstrated that the TIMP-2 (22000 Mr) expression level in colorectal carcinoma tissues was significantly lower than that in normal colorectal tissues. Furthermore, with the progression of tumor invasion depth, lymph node metastasis and tumor Duke’s stage, the TIMP-2 expression level was increased gradually, but did not reach the normal level. Our study also showed that there was an increase in the MMP-2/TIMP-2 ratio in colorectal carcinoma tissues compared to that in normal colorectal tissues. However, with the progression of tumor Duke’s stage, the MMP-2/TIMP-2 ratio was gradually decreased. We think that it is related to the increased TIMP-2 expression in the late stage. So we infer that the increased TIMP-2 expression in the late stage of colorectal carcinoma is a secondary change towards the increased MMP-2 activity.
Since the dynamic balance between MMP-2 and TIMP-2 is the decisive factor for the maintenance of ECM homeostasis and integrity, the balance disorder between them not only presents the regulation of colorectal carcinoma cells to ECM turnover, but also shows the influence of ECM on the behavior of tumor cells, which is important for colorectal carcinoma invasion and metastasis. During the progression of colorectal carcinoma, TIMP-2 plays a double role. On the one hand it inhibits MMP-2 activity, on the other hand, it is necessary for MMP-2 activation as a major cofactor, which has been reported in several studies[7,8]. In order to expand the growing space, colorectal carcinoma needs to enhance the MMP-2 activation and keep the ECM environment suitable for tumor cells, which must depend on TIMP-2 as a cofactor. In a word, the increase of TIMP-2 expression in the late stage of colorectal carcinoma predicts a worse prognosis.
| 1. | Curran S, Murray GI. Matrix metalloproteinases in tumour invasion and metastasis. J Pathol. 1999;189:300-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 2. | Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003;253:269-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 868] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 3. | Yoon SO, Park SJ, Yun CH, Chung AS. Roles of matrix metalloproteinases in tumor metastasis and angiogenesis. J Biochem Mol Biol. 2003;36:128-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 179] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Zucker S, Vacirca J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev. 2004;23:101-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 403] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 5. | Lynch CC, Matrisian LM. Matrix metalloproteinases in tumor-host cell communication. Differentiation. 2002;70:561-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 260] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3186] [Cited by in RCA: 3351] [Article Influence: 145.7] [Reference Citation Analysis (0)] |
| 7. | Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161-174. [PubMed] |
| 8. | Johansson N, Ahonen M, Kähäri VM. Matrix metalloproteinases in tumor invasion. Cell Mol Life Sci. 2000;57:5-15. [PubMed] |