Published online May 7, 2005. doi: 10.3748/wjg.v11.i17.2591
Revised: November 24, 2004
Accepted: December 9, 2004
Published online: May 7, 2005
AIM: To investigate the effect of probiotics supplemented by gut on the tight junctions of epithelial cells, barrier function and the microflora of rats with abdominal infection.
METHODS: After the model of cecal ligation and perforation established, SD rats were divided into two groups: parenteral nutrition (PN) group and PN+probiotics (probiotics) group, PN solution was supplemented by neck vein and probiotics was delivered via the jejunostomy tube for five days. Vena cava blood and the homogenated tissue of liver, lung and mesenteric lymph nodes were cultured to determine the bacterial translocation rate (BTR). The ultra-structure of epithelial tight junctions and microvilli of the gut were observed by electron microscopy; occluding expression was measured by indirect-immune fluorescence method; anaerobic bacterial growth by anaerobic culture and DNA fingerprint of bacterial colonies of the feces by PCR.
RESULTS: The quantity of lactobacteria and bifydobacteria in probiotics group was higher than that of PN group. The profiles of DNA fingerprint expression in probiotics group were similar to that in the normal group, a new 16S rDNA sequence appeared in the profile in PN group. The occludin expression, the integrality of the gut epithelial tight junction and microvilli in probiotics group were improved as compared with PN group. The BTR and endotoxin in blood were reduced more significantly in probiotics group as compared with PN group.
CONCLUSION: The probiotics could improve the gut microflora disturbance, increase occludin expression, maintain the gut epithelial tight junction and decrease the bacterial translocations rate.
- Citation: Qin HL, Shen TY, Gao ZG, Fan XB, Hang XM, Jiang YQ, Zhang HZ. Effect of lactobacillus on the gut microflora and barrier function of the rats with abdominal infection. World J Gastroenterol 2005; 11(17): 2591-2596
- URL: https://www.wjgnet.com/1007-9327/full/v11/i17/2591.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i17.2591
Since 1980s, Schmidt and Martindale have agreed upon the intestinal mucosa injury accomplished with bacterial and endotoxin translocation (BT & ET), followed by the development of endotoxemia, shock and multiple organ dysfunction syndrome (MODS), and the markedly increased death rate due to severe illness[1]. Despite developments in antisepsis and antibiotic prophylaxis, septic complications are common in surgical patients. It is known that the majority of postoperative infections are caused by gut derived organisms, some studies have focused their attention on the functioning of the gut mucosal barrier and the role of the indigenous gut microflora. There is good evidence from animal and in vitro studies that alteration of the gastrointestinal microflora with probiotic organisms can reduce the rate of BT to mesenteric lymph nodes. Although the significance of BT in humans remains to be determined, its postulated links with sepsis and multiple organ failure make it an obvious target for therapeutic intervention.
In recent years, scientific knowledge in the field of microbiology was expanded and it has been suggested that the gastrointestinal microflora has a role in maintaining human health[2]. The gastrointestinal ecology in normal subjects may be altered by administration of live micro-organisms, termed probiotics. These organisms have shown to inhibit the growth and adherence of potentially pathogenic bacteria to enterocytes, an important step in the process of BT. There is also an increasing evidence that probiotic organisms can interact with the gut-associated lymphoid tissue and influence the local mucosal and systemic immune function[3]. In addition, lactobacilli can secrete antimicrobial compounds called bacteriocins which inhibit a broad spectrum of enteric organisms. But, the mechanisms underlying the benefits derived from biotherapy with probiotics have not been sufficiently elucidated, with available data largely fragmentary and/or anecdotal. The present study was to determine the effect of probiotics on the micro-structure of tight junction and transmembrane protein-occludin expression, gut microflora and bacterial translocation by parenteral nutrition (PN) and probiotics supplemented by gut to the rats with abdominal infection.
Forty-nine SD rats weighing 250-320 g (FuDan University Medical Animal Center, Shanghai) were allowed ad libitum intake of water and standard rat food and subjected to alternate 12-h periods of dark and night. After 16-h fasting, all rats were anesthetized with intraperitoneal injections of 2% saline-ketamin injection liquid (30 mg/g weight). The rats were fixed in a supine position, and the neck, interscapular and abdominal region were shared and prepared in a sterile manner for catheterization and operation. The middle abdomen was opened with 4-cm incision, the abdominal infection model was established by cecum perforation and ligation (CPL)[4]. A silastic catheter (Ф1.2 mm) was placed by jejunostomy far away the cecum 30-40 cm, the catheter was tunneled abdominal subcutaneously to the back, and the abdominal incision was closed. A silastic catheter (0.6 mm inner diameter, 1.0 mm outer diameter) was inserted through the external jugular vein into the superior vena cava. The catheter was tunneled subcutaneously to the midscapular region and guarded by the flexible spring, and then hooked up to an infusion pump (B. Braun). The rats were maintained in individual metabolic cages[5]. The nutrient solution was induced at a constant infusion rate by the pump (2 mL/h) for 5 d during the study period. The trial was approved by our institutional animal committees.
After catheterization and the operation was over, all rats received intravenous 0.9% saline solution at 1.0 mL/h for 24-30 h to allow enough time to recover from the anesthesia. Rats were divided into two groups, group A (n = 7): received PN, the solution was infused averagely, the amount of infusion solution was about 80 mL/d during 1-5 d experimental period; group B (n = 8): PN +probiotic during 1-5 d, the probiotic was infused by jejunostomy tube about 10 mL/d. Group A and B were isonitrogenous and isocaloric (Table 1). The daily dose of amino acids was 2.5 g of nitrogen per kilogram, and the amount of nonprotein calories given was 250 kcal/(kg·d) as 50% glucose and 20% Intralipid (Energy Index 1:1). Multivitamins and electrolytes were also included in PN solution. The rats were killed on the 6th d in the morning for taking samples.
| Prescription | Dose |
| 50% Glucose (mL) | 33 8.5% |
| Novamin (mL) | 45 20% |
| Intralipid (mL) | 17 |
| Soluvita (mL) | 1 |
| Addamel (mL) | 1 |
| 10% KCl (mL) | 1 |
| 10% NaCl (mL) | 1 |
| RI (U) | 4 |
| Heparin (U) | 40 |
| Non-protein calorie (kJ) | 435 |
| (kcal) | 101 |
| Total nitrogen (mg) | 630 |
Probiotic containing live lactobacillus acidophilus (activity 1×108 cfu/mL) was supported by Shanghai Jiao Tong University Only Limited Company, and infused about 10 mL/day by jejunostomy tube thrice per day through 1-5 d.
The fecal sample (1 g) in cecum was placed in an anaerobic glove box within 1 h of collection and homogenized in prereduced brain-heart infusion broth and diluted from 10- to 10-8 fold. Portions (100 μL) of each dilution were spread onto the surfaces of plates which contained the following agar media and were incubated anaerobically at 37 °C: supplemented brucella blood agar (2 d, total anaerobic CFU), bacteroides bile esculin agar (2 d), egg yolk agar (after equal volumes of 95% ethanol were added to the dilutions and the preparations stood for 30 min to select for clostridial spores), and Rogosa SL agar (Difco) (2 d for lactobacilli and 4 d for bifidobacteria, after Lactobacillus colonies were marked at 2 d). The dilutions were removed from the anaerobic glove box and were used to inoculate (100 μL inocula) plates which contained the following media and were incubated aerobically at 37 °C: supplemented brucella blood agar (2 d, total aerobic CFU), MacConkey agar (Difco) (1 d, enterobacteria), bile esculin azide agar (Difco) (1 d, enterococci). To analyze the total Lactobacillus population, 10 colonies were picked randomly from a dilution agar plate containing about 100 colonies. The bacterium colonies were counted and identified by Microscan Autoscan-4 Machine (Dade Behringcom).
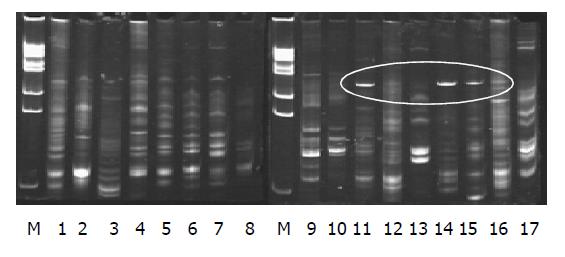
The fecal samples in cecum from each subject were also examined by PCR-denaturing gradient gel electrophoresis (DGGE) profiles. To extract bacterial DNA, 1 mL of fecal homogenate in pH 7.0 phosphate buffer (the buffer used for the azoreductase assay) was centrifuged at 14600 g for 5 min (5 °C). DNA was extracted from the resulting pellet with a Fast DNA kit (BIO 101, Vista, CA) by using CLS-TC (a cell lysis solution used for animal tissues and bacteria). The V2-V3 region of the 16S rDNA gene (positions from 339 to 539 in the Escherichia coli gene) of bacteria in the fecal samples was amplified by using primers bacteria ITS PS2 (5’-TG(C/T)ACACACCGCCCGT-3’), PL2 (5’-GGGT(G/C/T)CCCCATTC(A/G)G-3’). PCR was performed with 0.2-mL tubes by using a PCR Express thermal cycler (Hybaid, Teddington, UK). Each reaction mixture (50 μL) contained reaction buffer (10 Mm [final concentration] Tris-HCl, 2.5 mmol/L [final concentration] MgCl2, 50 mmol/L [final concentration] KCl [pH 8.3), each deoxynucleoside triphosphate at a concentration of 200 μmol/L, 20 pmoL of each primer, 1 μL of fecal DNA, and 2.5 U of Taq DNA polymerase (Boehringer, Mannheim, Germany). The following amplification program was used: 94 °C for 3 min, 30 cycles consisting of 94 °C for 30 s, 56 °C for 30 s, and 68 °C for 60 s, and then 7 min at 68 °C. DGGE was performed by using a DCode universal mutation detection system (Bio-Rad, Richmond, CA) and gels that were 16 cm×16 cm×1 mm; 6% polyacrylamide gels were prepared and electrophoresed with 1×TAE buffer prepared from 50×TAE buffer (2 mol/L Tris base, 1 mol/L glacial acetic acid, 50 mmol/L EDTA). The denaturing gradient was formed by using two 6% acrylamide (acrylamide/bisacrylamide ratio, 37.5:1) stock solutions (Bio-Rad). The gels contained a 22-55% gradient of urea and formamide that increased in the direction of electrophoresis. A 100% denaturing solution contained 400 mL/L formamide and 7.0 mol/L urea. Electrophoresis was performed at 130 V (constant voltage) and 60 °C for about 4.5 h. Electrophoresis was stopped when a xylene cyanol dye marker reached the bottom of a gel. The gels were stained with an ethidium bromide solution (5 μg/mL) for 20 min, washed with deionized water, and reviewed by UV transillumination[13].
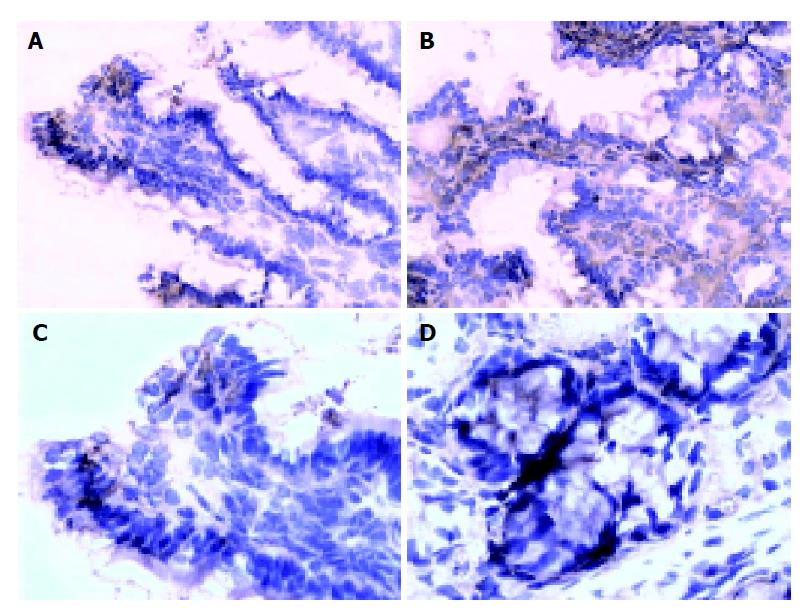
The distal ileum and colon tissue were taken to determine the occludin expression by indirect- immune fluorescence. Frozen tissue was cut into 10-µm sections, dried on positively charged slides, washed with PBS, and incubated at 37 °C for 30 min with 10% normal goat serum. Excess serum was blotted, and the slides were incubated with a 1/50 dilution of primary antibody for 1 h at 37 °C. The first antibody was sheep- anti-rats occludin antibody (Santa Cruz). The slides with the second antibody anti-sheep IgG (Santa Cruz) were then incubated with FITC-conjugated goat anti-rabbit IgG (1/20 dilution) for 30 min at 37 °C and washed again with PBS. All slides were then mounted with 0.1% p-phenylenediamine in PBS-glycerol and coded before examination. Treated sections were examined with UV light in an incident-light fluorescent microscope, and the fluorescence pattern (focal, diffuse; mesangial, loop) was defined. Fluorescence intensity was graded as negative, or 1+ through 4+. Control sections lacking primary antibody were included with each experiment. All sections were examined by two authors (Shen TY and Zhang HZ) independently. The data were analyzed by HPIAS1000 high definition color image manipulation system. Under the same expanding multiple (400), occludin density was determined per field of vision by light-densimeter (at least five fields per slide).
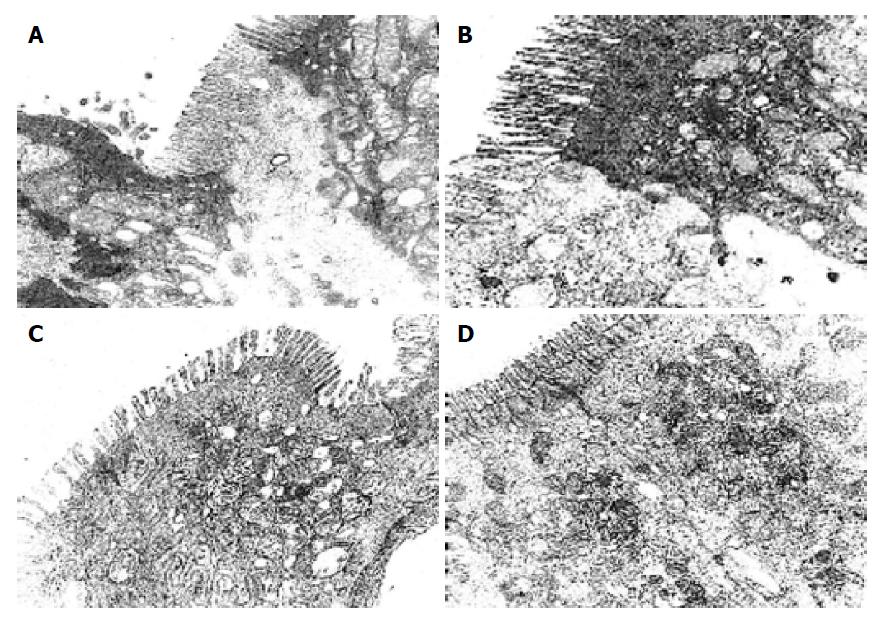
One fixation procedure was used for conventional thin-section electron microscopy. The fixation procedure involved incubation with OsO4 alone (1% or 2% in phosphate buffer) at 0 °C for 30 min. After fixation, the ileum and cecum were washed extensively in Veronal acetate buffer (90 mmol/L, pH 6.0), stained by incubation at 0 °C for 60 min in uranyl-magnesium acetate (0.5%) in the same buffer, washed again, dehydrated, and embedded. Thin sections were doubly stained with uranyl acetate and lead nitrate and examined to observe the change of tight junction and microvilli (2×104) by Philip EM 400 electron microscopes.
One milliliter blood from vena cava, 5 g tissue of mesenteric lymphoside, liver and lung tissue, were collected, respectively, and homogenized in a tube containing cardio-cerebral leachate that was incubated at 35 °C; after 18-24 h, methylene blue-eosin staining method was adopted, and continued to be cultured at 35 °C; after 18-24 h, the number of bacterium were counted.
Analysis of data was performed using χ2 and t analysis, the experimental data were expressed as mean±SD of the sample and comparison between treatment groups using one-way analysis of variance. P value below 0.05 was considered statistically significant.
The CPL model was established successfully in 49 SD rats, the total death rate during 6 d was 69.3%; the death rate in PN group (78.7%) was higher than that in probiotics group (50%, χ2 = 4.204, P = 0.0403<0.05, Table 2).
There were no marked differences of the gut bacterial strains in the probiotics group as compared with PN group, P>0.05. But the amount of lactobacteria and bifidobacteria increased, and the amount of enterococci decreased in probiotics group as compared with PN group, P<0.05 (Table 3).
The electrophoresis appearance of DNA sequence of the profiles in PN group (lane 11-17) were less than that in the control group (lane 9-10); lane 11, 14, 15 appeared of a new 16S rDNA sequence in the profile (labeled in Figure 1). The sequence of electrophoresis expression in probiotics group (lane 1-8) was similar to that in the control group (lane 9-10).
There are more occludin expressions in the probiotics group in the surface, and intracellular and cell-internal of the epithelial cells as compared with PN group (Figures 2A-2D). The occludin positive expression area per measured-window in probiotics group was higher than that in PN group (t = -4.436, P = 0.001<0.01, t = -2.429, P = 0.036<0.05, Table 4).
There were more integrated tight junctions, less mitochondrion endoplasm and micro-villi brushed in probiotics group as compared with PN group (Figures 3A-3D). The readability of the tight junction in probiotics group was clearer than that in PN group.
Bacterium translocation rate (BTR) in PN group (60.7%) was higher than that in probiotics group (34.3%, χ2 = 4.1625, P = 0.0413<0.05, Table 5).
The role of commensal and probiotic bacterial in the physiology of the gastrointestinal tract is not completely understood. Probiotics are defined as “living organisms, which upon ingestion in certain numbers exert health benefits beyond inherent basic nutrition”. The importance of a “health” gut microbiota has been recognized for a long time but only recently specific attention has been focused on the potential of probiotics as preventative and therapeutic agents in gastrointestinal diseases. The use of probiotics in animal models of inflammatory bowel disease and in diarrhea of premature infants, severe burn patients, and acute and chronic colitis has shown potential beneficial effects of probiotic Lactobacilli, Bifydobacteria, and Saccharomyces. It is known that stimulation by commensal bacterial antigens is crucial for the normal development of the mucosal immune system and maintenance of tolerance. Moreover, probiotics have shown to attenuate or abolish tumor necrosis factor α stimulated interleukin 8 production in intestinal epithelial cells. The studies described here show in addition that, by diverse mechanisms, living probiotics are able to prevent or counteract the full spectrum of epithelial dysfunction induced by an enteroinvasive pathogen. In the recent years, more attention has been taken in the probiotics practice, and has gained some certain results. Eizaguirre et al[6], gave Wistar rats with 80% intestinal resection infusion probiotics, and decreased the gut bacterial translocation rate (BTR) to 43%. Olah et al[7], reported that probiotics were used in the patients with acute pancreatitis, and markedly decreased pancreatic second infection rate and hospital stay time. Rayes et al[8], reported that patients with post-surgical severity that use probiotics (L. plantarum 299, 109 cfu/d), might decrease postoperative infection rate and shorten the time using antibiotics. However, the data in this area are relatively sparse, and the detailed mechanism based investigations of efficacy are largely lacking.
Traditionally, the fecal microflora has been analyzed by using bacteriological culture methods. It has been claimed that approximately 88% of the total microscopic counts of bacterial cells can be cultivated from feces when appropriate techniques are employed[9]. Our fecal anaerobic culture results showed that there were no marked differences of the gut bacterial strains in the probiotics group as compared with PN group, P>0.05. But the amount of lactobacteria and bifydobacteria is increased, and the amount of enterococci decreased in probiotics group as compared with PN group, P<0.05. However, because the conditions of the anaerobic culture was limited, only some local bacterium were determined by quantitative analysis, and could not reflect the whole profiles of gut preponderant bacterium colony. Fortunately, analysis of terrestrial and aquatic ecosystems in more recent years has benefited from the use of molecular biological methods with which community profiles have been established. The molecular methods involve the amplification by polymerase chain reactions (PCR) of 16S ribosomal RNA genes (16S rDNA) from microbial DNA extracted from samples collected from particular habitats[10,11]. It is important to note that the PCR-based and oligonucleotide probe methods outlined above could differentiate between strains belonging to the same bacterial species. Summaries of useful molecular typing (genetic DNA fingerprinting) methods provided direct response to the gut bacterial strains balance[11-13]. We measured the impact of consumption of this probiotic on the fecal microflora by using PCR-DGGE, the results showed that the electrophoresis DNA fingerprint expression reflecting the gut bacterium variety in probiotics group were similar to the change of DNA fingerprint of the control group, for rats that received PN support the new 16S rDNA sequence appeared, and suggested that gut flora imbalance existed. Our results suggested that in rats that received PN a serious gut flora imbalance occurred, but the gut microflora could be improved using probiotics.
The tight junctions of intestinal epithelial cells form the most apical component of the junctional complex and create a selective permeability barrier along the paracellular pathway. Some experimental evidence suggests that occludin may be vital in a functional capacity. It is reported that this complex tight junction allows the paracellular diffusion of ions and small solutes but excludes potentially toxic macromolecules and micro-organisms, the permeability characteristics of a given barrier can be dynamically altered by a variety of physiological, pathological, and pharmacological stimuli. It has reached the same recognition in international medicine that change of the occludin protein presents not only the injury extent of tight junction under the pathogenesis but also indicate the extent recovered from injury[14-17]. The present study of occludin expression by indirect-immune fluorescence showed that there are more occludin expression of the surface, intracellular and cell-internal of the epithelial cells in probiotics group as compared with PN group. The occludin positive expression area per measured-window in probiotics group was higher than that in PN group. The expression of occludin increased as the gut bacterium colony improved, moreover, the impact of occluding expression on the colon was more effective than that on the intestine in probiotics group. This result was consistent with bacterium fixed in the colon and physiological actions. To better understand the ultra-structure of TJ, the primary results by EM found that the intestinal micro-villi were slightly brushed off, mitochondrion endoplasm vacuole and obvious tight junction both of intestinal and colon in probiotics group as compared with PN group; meanwhile, the BTR of MLN and the remote organ in probiotic group were less than that in PN group, the death rate in PN group was higher than that in probiotics group. So, the prognostic of the rats that received probiotics showed a marked improvement.
In summary, probiotics increased the expression of transmembrane binding protein, maintain the structure of tight junction, improved the gut bacterium colony disorder in the rats with abdominal infection, decreased the BTR,and protected the barrier function. Under PN supporting, it is easy to induce the gut bacterium disorder and mucosa barrier injury, however, the gut barrier function could be improved by using probiotics.
| 1. | Schmidt H, Martindale R. The gastrointestinal tract in critical illness: nutritional implications. Curr Opin Clin Nutr Metab Care. 2003;6:587-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Filho-Lima JV, Vieira EC, Nicoli JR. Antagonistic effect of Lactobacillus acidophilus, Saccharomyces boulardii and Escherichia coli combinations against experimental infections with Shigella flexneri and Salmonella enteritidis subsp. typhimurium in gnotobiotic mice. J Appl Microbiol. 2000;88:365-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Link-Amster H, Rochat F, Saudan KY, Mignot O, Aeschlimann JM. Modulation of a specific humoral immune response and changes in intestinal flora mediated through fermented milk intake. FEMS Immunol Med Microbiol. 1994;10:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 226] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 4. | Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock--a review of laboratory models and a proposal. J Surg Res. 1980;29:189-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1087] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 5. | Qin HL, Su ZD, Hu LG, Ding ZX, Lin QT. Effect of early intrajejunal nutrition on pancreatic pathological features and gut barrier function in dogs with acute pancreatitis. Clin Nutr. 2002;21:469-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (4)] |
| 6. | Eizaguirre I, Urkia NG, Asensio AB, Zubillaga I, Zubillaga P, Vidales C, Garcia-Arenzana JM, Aldazabal P. Probiotic supplementation reduces the risk of bacterial translocation in experimental short bowel syndrome. J Pediatr Surg. 2002;37:699-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Oláh A, Belágyi T, Issekutz A, Gamal ME, Bengmark S. Randomized clinical trial of specific lactobacillus and fibre supplement to early enteral nutrition in patients with acute pancreatitis. Br J Surg. 2002;89:1103-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 292] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 8. | Rayes N, Seehofer D, Müller AR, Hansen S, Bengmark S, Neuhaus P. Influence of probiotics and fibre on the incidence of bacterial infections following major abdominal surgery - results of a prospective trial. Z Gastroenterol. 2002;40:869-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Moore WE, Holdeman LV. Special problems associated with the isolation and identification of intestinal bacteria in fecal flora studies. Am J Clin Nutr. 1974;27:1450-1455. [PubMed] |
| 10. | Franks AH, Harmsen HJ, Raangs GC, Jansen GJ, Schut F, Welling GW. Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl Environ Microbiol. 1998;64:3336-3345. [PubMed] |
| 11. | Fernández MF, Boris S, Barbés C. Probiotic properties of human lactobacilli strains to be used in the gastrointestinal tract. J Appl Microbiol. 2003;94:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 276] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 12. | Tilsala-Timisjärvi A, Alatossava T. Characterization of the 16S-23S and 23S-5S rRNA intergenic spacer regions of dairy propionibacteria and their identification with species-specific primers by PCR. Int J Food Microbiol. 2001;68:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | García-Martínez J, Acinas SG, Antón AI, Rodríguez-Valera F. Use of the 16S--23S ribosomal genes spacer region in studies of prokaryotic diversity. J Microbiol Methods. 1999;36:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 149] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Hang XM, Yang H, Corne W. PCR amplification of anaerobic fungal 18s rDNA from landfill sites. Chin J Biotech. 2001;17:515-519. |
| 15. | Nishiyama R, Sakaguchi T, Kinugasa T, Gu X, MacDermott RP, Podolsky DK, Reinecker HC. Interleukin-2 receptor beta subunit-dependent and -independent regulation of intestinal epithelial tight junctions. J Biol Chem. 2001;276:35571-35580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Nusrat A, Chen JA, Foley CS, Liang TW, Tom J, Cromwell M, Quan C, Mrsny RJ. The coiled-coil domain of occludin can act to organize structural and functional elements of the epithelial tight junction. J Biol Chem. 2000;275:29816-29822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 155] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Berkes J, Viswanathan VK, Savkovic SD, Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut. 2003;52:439-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 452] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
Science Editor Guo SY Language Editor Elsevier HK