Published online May 7, 2005. doi: 10.3748/wjg.v11.i17.2574
Revised: November 9, 2004
Accepted: December 21, 2004
Published online: May 7, 2005
AIM: To investigate the inhibitive effect of hepatitis B virus (HBV)-TRL on HBV replication.
METHODS: Based on previously constructed pcDNA3.1(-)/TRL, TR, TRmut, HBV core protein (HBVc) and hEDN, interest gene sequences TRL, TR, HBVc and hEDN were inserted into adenovirus shuttle plasmid pDC316 respectively and co-transfected HEK293 cells with rescue plasmid pBHGlox(delta)E1,3Cre to acquire RAd/TRL, TR, HBVc and hEDN. And then RAds were identified, amplified and the titers in HEK293 cells were determined. RAd/TRL and TR were named as the experimental groups, and others were control ones. After HepG2.2.15 cells were infected, RAd/TRL expression was identified by indirect immunofluorescence staining. Supernatant HBV-DNA content was determined by fluorescent quantification PCR. Meanwhile, metabolism of HepG2.2.15 cells was evaluated by MTT colorimetry.
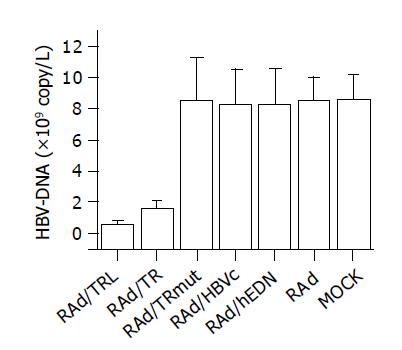
RESULTS: RAd vectors with distinct interest gene sequence were successfully constructed. Effective expression of RAd/TRL in HepG2.2.15 cells resulted in a significant decrease of supernatant HBV-DNA content compared to RAd/TR (0.63±0.14 vs 1.60±0.47, P = 0.0266, <0.05) and other control groups (0.63±0.14 vs 8.50±2.78, 8.25±2.26, 8.25±2.29, 8.50±1.51, 8.57±1.63, P<0.01). MTT assay suggested that there were no significant differences in cell metabolic activity between groups (P>0.05).
CONCLUSION: The construction and expression of RAd/TRL has been achieved and it could inhibit HBV replication successfully, which has laid the foundation for further research on anti-HBV activity in vivo.
- Citation: Gong WD, Zhao Y, Yi J, Ding J, Liu J, Xue CF. Anti-HBV activity of TRL mediated by recombinant adenovirus. World J Gastroenterol 2005; 11(17): 2574-2578
- URL: https://www.wjgnet.com/1007-9327/full/v11/i17/2574.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i17.2574
Hepatitis B virus (HBV) infection remains a major public health problem worldwide[1-4]. HBV causes transient and chronic infection of liver. Transient infection may produce serious illness, and chronic infection may also have serious consequences: nearly 25% (350 million) of chronic HBV-infected patients end with untreatable liver cancer[5-7]. The available treatments are of limited efficacy, such as interferon-α (INF-α)[8-11], nucleoside analog[12-15],and gene therapy strategy[16]. Alternative approaches to inhibit HBV replication are urged. Capsid-targeted viral inactivation (CTVI, also called virion-targeted viral inactivation) was established by Natsoulis and Boeke in 1991, in which a viral capsid protein or other virion-associated protein as a carrier guided a degradative enzyme into virus particles specifically to inhibit virus replication or kill it[17]. CTVI has been thoroughly investigated in experimental treatment for retrovirus, such as Moloney murine leukemia virus and dengue virus, showing a promising prospect as an antiviral treatment[18,19]. Based on CTVI, Beterams[20] found that SN and HBV core protein (HBVc) fusion protein can inhibit HBV replication effectively. Previously we fused HBVc to human eosinophil-derived neurotoxin (hEDN), and after transfection of the fusion protein encoding plasmid into HepG2.2.15 cells, HBV replication was inhibited, due to the fact that HBV pregenome RNA (pgRNA) was degraded by the effector molecule, hEDN, which was guided by the target molecule, HBVc[21,22]. And then we also reported the further enhancement of the degradative effect by introduction of a linker sequence (Gly4Ser)3 to separate the effector molecule and the target one, which was named as TRL mediated by eukaryotic vector pcDNA3.1(-)[23]. In this work we report the anti-HBV effect of TRL mediated by recombinant adenovirus (RAd) as the alternative vector in order to further use TRL in vivo to evaluate its function.
pcDNA3.1(-)/TRL, TR, HBVc, hEDN were constructed in our laboratory[25]. HepG2.2.15 cells were kindly provided by Dr. Hao (Department of Infection, Tangdu Hospital, Fourth Military Medical University). HEK293 cells and blank vector recombinant adenovirus (RAd, titer: 5.3×107 nfu/L) were kindly provided by Dr. Zhang (Department of Microbiology, Fourth Military Medical University). Polyclonal rabbit anti-TR sera were produced by Dr. Zhao[24]. AdMax Kit D were purchased from Canada Microbix Biosystem Company. Restriction enzymes BglII, EcoRI and HindIII, Takara Ex TaqTM, DNA marker-DL2000 and T4 DNA ligase were purchased from Takara Biotechnology Co., Ltd. Plasmid Miniprep kit and Agarose Gel Extraction kit were purchased from Watson Biotechnology, Shanghai, DMEM and fetal calf serum (FCS) were purchased from Gibco Company; HBV-DNA fluorescent quantification PCR (FQ-PCR) reagent was purchased from Amplly Bio-company, Ximeng. Rabbit anti-mouse IgG labeled with FITC was purchased from Sino-American Biotechnology Company, Henan. Reagents for calcium phosphate transfection were purchased from Sigma. GeneAmp PCR System 9600 was purchased from Perkin Elmer, USA.
HepG2.2.15 cells integrated full-length HBV genome were cultured in DMEM media containing 150 mL/L FCS at 37 °C in 50 mL/L CO2. G418 was added to screen cells at the final concentration of 100 g/L. The media were freshened once in every 2 d and the cells were passaged every 6 d. HEK293 cells were maintained in DMEM supplemented with 100 mL/L FCS, 50 kU/L penicillin and 50 mg/L streptomycin at 37 °C in 50 mL/L CO2.
pcDNA3.1(-)/TRL, TR, TRmut, HBVc and hEDN bearing BamHI and HindIII restriction sites were digested; the interest fragments TRL, TR, TRmut, HBVc and hEDN were then subcloned into shuttle plasmid pDC316, which was restricted by BglII and HindIII to produce plasmid pDC316/TRL, TR, TRmut, HBVc and hEDN. The five plasmids were confirmed by EcoRI and HindIII digestion and analyzed by electrophoresis in 12 g/L agarose gel.
The transfection work was performed according to the manufacturer’s instructions. Approximately 1.0×106 HEK293 cells were seeded in 60-cm plates 24 h before transfection, by that time they reached 70% confluency. Twenty micrograms of shuttle plasmid, 6 μg rescue plasmid pBHGlox(delta)E1,3Cre and 60 μL 2 mol/L CaCl2 were added to a tube and mixed well, then DDW was added to a total volume of 300 μL. Three hundred microliters of 2×Hepes buffered saline was added slowly to the tube while being constantly mixed. And they were set at room temperature for 30 min to form the precipitate. Afterwards the media were aspirated thoroughly and replaced by DMEM without FCS. The cells were returned to the incubator for at least 20 min to let the precipitate to get distributed evenly over the plates. Then the precipitate was mixed with media by gently agitating and was returned immediately into incubator for another 16 h. The cells were washed twice with HBS after the media were removed. The cells were then fed with the complete media. The plates were monitored daily for the appearance of cytopathic effect (CPE), by which the cells would appear round and refractile and would begin to lift off the surface of the plate (usually after 36-48 h). When >90% of the cells showed CPE (usually after 72 h), cells were harvested in their culture media and subjected to three freeze (methanol/dry ice bath)/thaw(37 °C waterbath) cycles. After the cell debris was sedimented, supernatant containing the adenovirus particles was the RAd stocks and stored in small aliquots at -70 °C after 10% glycerol added for further identification.
Thus five RAds, which were RAd/TRL, RAd/TR, RAd/TRmut, RAd/HBVc, RAd/hEDN, were obtained. Two methods were here to identify the acquired RAds: (1) infective identification. The RAds could be preliminarily confirmed if HEK293 cells (70% confluency) showed typical CPE after infected by the RAds; (2) PCR to amplify the interest gene fragments of RAds. Procedures for PCR identification were described in Ref. 28. The amplification products were analyzed by electrophoresis in 10 g/L agarose gel.
According to the manufacturer’s instructions, 1.0×106 HEK293 cells were seeded in 60-cm dishes 24 h before infection, by which time they reached 70% confluency. Aspirate the media from dishes, add 1 mL of media/RAd solution to each plate and return to incubator for 90 min. Then add another 2 mL media and continue to incubate. Cells were harvested as above when >50% cells show typical CPE. RAd stocks were stored at -70 °C for further titer determination.
Approximately 104/well HEK293 cells were seeded in 96-well plates 24 h before infection. Procedures for titer determination by terminal dilution are described in Ref. 28. RAd stocks were stored at -70 °C for further effect evaluation.
RAd titer to infect HepG2.2.15 cells was 10 pfu/cell according to manufacturer’s instructions. Approximately 105 HepG2.2.15 cells/well were seeded in 6-well plates 24 h before infection. The experiment was divided into three groups: test group in which RAd/TRL was used, and two control groups in which blank vector RAd was used in the second group and the third was mock infection. Tri-wells were contained in each group. Polyclonal rabbit anti-TR sera were used as the first antibody[24], and as a second antibody, rabbit anti-mouse IgG labeled with FITC was used. The time chosen for indirect immunofluorescence staining was 24, 36, 48 and 72 h post-infection, respectively.
Twenty-four hours before infection, HepG2.2.15 cells were plated into a 24-well plate with a density of 105 cells/well. Infections were performed as described above. To determine HBV-DNA content, infection experiment was divided into seven groups; they were RAd/TRL, RAd/TR, RAd/TRmut, RAd/HBVc, RAd/hEDN, blank RAd and mock infection, named as A to G respectively, among which A and B groups were testing group; the others were control ones. Each infection was performed in triplicate. Forty-eight hours post-infection the cells suspension was taken and HBV-DNA content was quantified by FQ-PCR. Meanwhile, the infected cells were used to analyze the metabolic activity in order to analyze the effect of expressing protein to host cells. The data obtained were analyzed by SPSS software.
Metabolism of cells was evaluated by MTT colorimetry. Forty-eight hours following infections, 20 μL of MTT solution (5 g/L) was added into each well and incubated at 37 °C for another 4 h. One hundred and fifty milliliters of DMSO was added and surged for 10 min to dissolve the crystal completely. Absorbance values were identified at 490-nm wavelength by ELISA reader.
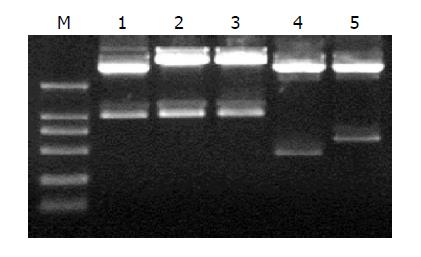
The five plasmids pDC316/TRL, TR, TRmut, HBVc and hEDN were confirmed by restrictions of EcoRI and HindIII and were analyzed by electrophoresis in 12 g/L agarose gel. Gene sequences of interest are shown in Figure 1. The results suggested that the construction was successful.
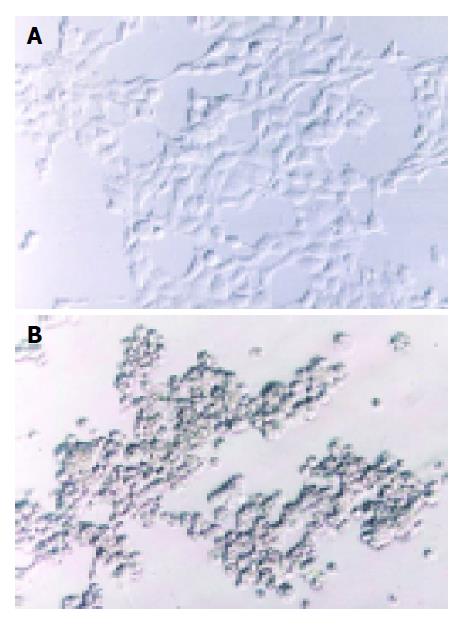
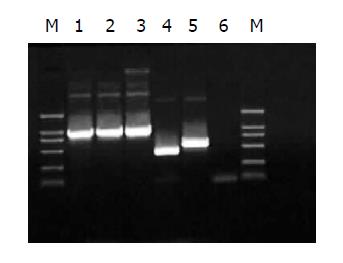
Two methods were here to identify the RAds: (1) infective identification: Typical CPE showed after co-transfecting shuttle plasmids with rescue plasmid pBHGlox(delta)E1,3Cre to HEK293 cells for 7 d (Figure 2); (2) PCR to amplify the interest gene fragments of RAds. The amplification products were analyzed by electrophoresis in 10 g/L agarose gel. The results suggested that the RAds were produced successfully (Figure 3).
Titer determination by terminal dilution of the five RAds, RAd/TRL, TR, TRmut, HBVc and hEDN, was 4.1×106, 4.5×106, 3.7×106, 6.3×106, and 5.6×106 nfu/L, respectively.
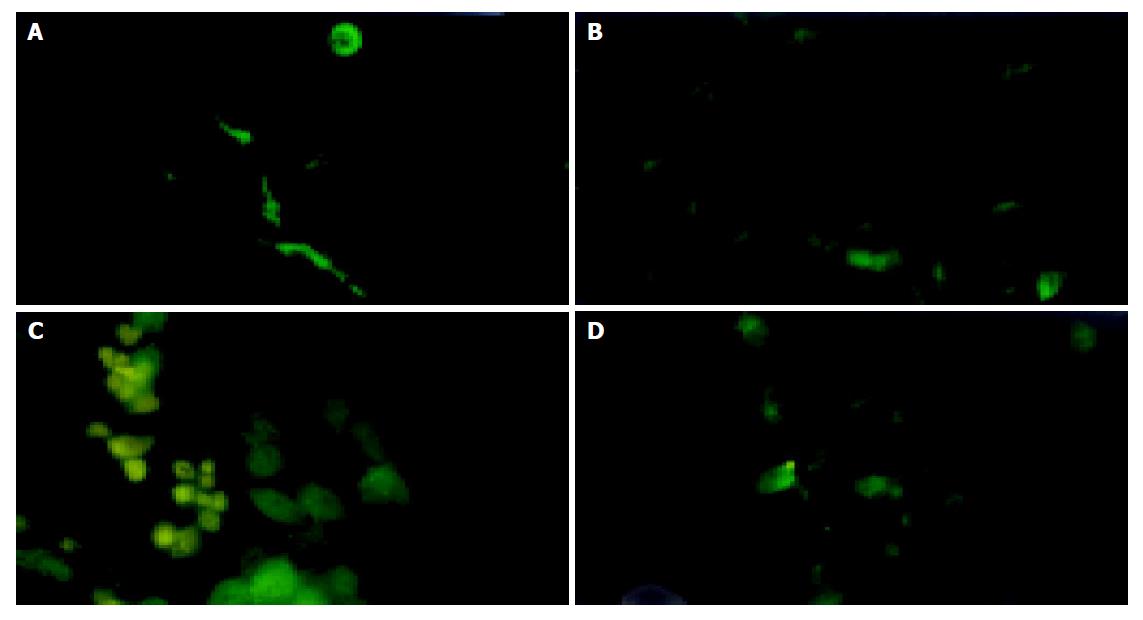
The expression of TRL fusion protein of RAd/TRL in HepG2.2.15 cells could be observed as 24, 36, 48 and 72 h post-infection respectively and the strongest expression was 48 h post-infection (Figure 4).
HBV-DNA content of HepG2.2.15 cells supernatant was determined by FQ-PCR after infection to analyze the anti-HBV activity of RAd/TRL. The significant decrease of HBV-DNA content in RAd/TRL (A) group, compared to RAd/TR (B) (P = 0.0266, <0.05), suggested that linker introduction enhanced anti-HBV activity, which may be due to optimization of the folding of both hEDN and HBVc molecules[23]. Also there were significant differences between groups A, B and groups C-G (P<0.01), and no significant difference was found between control groups C-F and group G (P>0.05). Compared to mock infection group, the supernatant HBV-DNA content of RAd/TRL group declined by about 59.9% (Figure 5).
Forty-eight hours post-transfection, cell growth was observed and cell toxicity effect of TRL fusion protein to HepG2.2.15 cells was detected by MTT assay. The A490 value of HepG2.2.15 cells infected with RAd/TRL, RAd/TR, RAd /TRmut, RAd/HBVc, RAd/hEDN, RAd and mock infection was 0.67±0.09, 0.69±0.09, 0.69±0.09, 0.65±0.06, 0.77±0.10, 0.76±0.14, 0.62±0.06, respectively (mean±SD, n = 3). The results suggested that there were no significant differences between groups (P>0.05).
HBV infection was an important health problem worldwide. Analog of interferons and nucleosides were effective drugs for chronic HBV infection, but only 20-30% of treated patients maintained a long-lasting response to anti-viral drugs[26]. The expense of prolonged treatment made these therapies poorly suitable for people in developing countries, where the prevalence of chronic HBV infection was often high. Therefore, new therapy strategy for HBV infection was urged.
In the year 1991, Natsoulis[17] established an anti-virus strategy, CTVI, which was to guide effector molecule to target molecule and inhibit virus replication. CTVI had been thoroughly investigated in experimental treatment for retrovirus, showing a promising prospect as an antiviral treatment[19-21]. Based on CTVI, Liu[21] constructed a eukaryotic expression vector pcDNA3.1(-), which fused HBVc to human eosinophil-derived neurotoxin (hEDN), and after transfection into HepG2.2.15 cells HBV replication was inhibited. Meanwhile, Ding[22] constructed TAT-TR fusion protein, which inhibited HBV replication successfully via protein transduction domain TAT, and they had no side-effects to the host cells. To further enhance the inhibition effect of TR, the classic linker (Gly4Ser)3 was introduced to separate the effector molecule (HBVc) and the target one (hEDN); using eukaryotic expression vector pcDNA3.1(-) to transfect HepG2.2.15 cells, the result showed that TRL was more powerful to inhibit HBV replication than TR. The effect of linker was elucidated in Ref. 26. Further the more safety vector in gene therapy, RAd, was used as an alternative for in vivo gene delivery. Adenovirus-mediated genome transfer had distinct advantages. For example, adenovirus could transfer genes to a broad spectrum of cell types, and gene transfer was not dependent on active cell division. Adenovirus vectors were therefore extremely useful for both in vitro and in vivo studies in basic biology. Of known gene delivery vectors, it most efficiently transferred foreign DNA into the livers of a broad variety of experimental animals. In the liver, it predominantly infected hepatocytes. Additionally, high titers of viruses and high levels of transgene expression generally could be obtained[27].
As a result, we chose RAd vector instead of vector pcDNA3.1(-) to infect HepG2.2.15 cells directly in order for the efficient expression of transgene. In our investigation, supernatant HBV-DNA content determined by FQ-PCR showed that there was a significant decrease from TRL group compared to other negative control groups (P<0.01), no significant decrease among negative control groups (P>0.05); a decrease between TRL and TR group was observed (P = 0.0266, <0.05), the supernatant HBV-DNA content of TRL group declined by about 59.9%. The above results hinted that the recombinant adenoviral vector had a stronger effect of transgene expression than that of eukaryotic expression that was why we chose RAd other than the eukaryotic expression vector. And meanwhile the results confirmed again that TRL was more powerful to inhibit HBV replication than that of TR. MTT results suggested that the fusion protein had no side-effect on metabolism of cells (P>0.05).
Two traditional methods based on homologous recombinant were there to construct recombinant adenovirus. One was two plasmids co-transfection: shuttle plasmid with interest gene and helper plasmid with adenovirus genome co-transfect HEK293 cells. Then recombinant adenovirus vector would be created by homologous recombining of these two plasmids and packaging virus using HEK293 cells; the other was Ad-easy system: the cDNA of interest was first cloned into a transfer vector, the resulting plasmid was co-transformed into E. coli BJ5183 with most of adenovirus genome; recombinants obtained by Cre recombinase of bacteria were selected with kanamycin and screened by restriction enzyme analysis; the recombinant adenoviral construct was then transfected into HEK293 cells to produce viral particles. But these two methods had all shortcomings. The first had low homologous recombinant efficient and as for the second, the procedures were too complex. AdMax system was used in this research, which had shuttle plasmid with interest gene and LoxP site, and helping plasmid with Cre recombinase sequence, LoxP site and most of adenovirus genome co-transfect HEK293 cells. Then recombinant adenovirus vector would be created by Cre recombinase. This method had high recombinant efficiency and was comparatively simple[28,29].
Therefore, we concluded that TRL mediated by RAd vector could be expressed in cells and could inhibit HBV replication, which laid the foundation for using TRL in the therapy of HBV infection in vivo.
| 1. | Fleming J. Current treatments for hepatitis. J Infus Nurs. 2002;25:379-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | elSaadany S, Tepper M, Mao Y, Semenciw R, Giulivi A. An epidemiologic study of hepatocellular carcinoma in Canada. Can J Public Health. 2002;93:443-446. [PubMed] |
| 3. | Hassan MM, Hwang LY, Hatten CJ, Swaim M, Li D, Abbruzzese JL, Beasley P, Patt YZ. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 539] [Article Influence: 22.5] [Reference Citation Analysis (1)] |
| 4. | Torbenson M, Thomas DL. Occult hepatitis B. Lancet Infect Dis. 2002;2:479-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 400] [Article Influence: 16.7] [Reference Citation Analysis (1)] |
| 5. | Lok AS. Prevention of hepatitis B virus-related hepatocellular carcinoma. Gastroenterology. 2004;127:S303-S309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Mazumdar TN. Management of chronic hepatitis B infection: an update. J Indian Med Assoc. 2001;99:306-308, 310. [PubMed] |
| 7. | Chen N, Zhu C, Hu D, Zeng F. The clinical significance of negative serological markers of hepatitis B infection in hepatitis B virus carriers with chronic hepatic disease. Zhonghua NeiKe ZaZhi. 2002;41:653-655. [PubMed] |
| 8. | Hino O, Kajino K, Umeda T, Arakawa Y. Understanding the hypercarcinogenic state in chronic hepatitis: a clue to the prevention of human hepatocellular carcinoma. J Gastroenterol. 2002;37:883-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Esteban R. Management of chronic hepatitis B: an overview. Semin Liver Dis. 2002;22 Suppl 1:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Wai CT, Chu CJ, Hussain M, Lok AS. HBV genotype B is associated with better response to interferon therapy in HBeAg(+) chronic hepatitis than genotype C. Hepatology. 2002;36:1425-1430. [PubMed] |
| 11. | Manns MP. Current state of interferon therapy in the treatment of chronic hepatitis B. Semin Liver Dis. 2002;22 Suppl 1:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Shindo M, Hamada K, Koya S, Sokawa Y, Okuno T. The clinical significance of core promoter and precore mutations during the natural course and interferon therapy in patients with chronic hepatitis B. Am J Gastroenterol. 1999;94:2237-2245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Liaw YF. Therapy of chronic hepatitis B: current challenges and opportunities. J Viral Hepat. 2002;9:393-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Wolters LM, Hansen BE, Niesters HG, de Man RA. Viral dynamics in chronic hepatitis B patients treated with lamivudine, lamivudine-famciclovir or lamivudine-ganciclovir. Eur J Gastroenterol Hepatol. 2002;14:1007-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Song JW, Zhang G, Lin JG, Tang WX, Lin JS. Clinical study of lamivudine and interferon combinate administration to inhibit hepatitis B virus replication. Zhonghua GanZangBing ZaZhi. 2004;12:593-596. [PubMed] |
| 16. | Chiou HC, Lucas MA, Coffin CC, Banaszczyk MG, Ill CR, Lollo CP. Gene therapy strategies for the treatment of chronic viral hepatitis. Expert Opin Biol Ther. 2001;1:629-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Natsoulis G, Boeke JD. New antiviral strategy using capsid-nuclease fusion proteins. Nature. 1991;352:632-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Schumann G, Hermankova M, Cannon K, Mankowski JL, Boeke JD. Therapeutic effect of a Gag-nuclease fusion protein against retroviral infection in vivo. J Virol. 2001;75:7030-7041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Qin CF, Qin ED. Development of cell lines stably expressing staphylococcal nuclease fused to dengue 2 virus capsid protein for CTVI. Acta Biochim Biophys Sin (Shanghai). 2004;36:577-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Beterams G, Nassal M. Significant interference with hepatitis B virus replication by a core-nuclease fusion protein. J Biol Chem. 2001;276:8875-8883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 21. | Li YH, Liu J, Xue CF. Construction of HBV targeted-ribonuclease and ies expression in 2.2.15 cell line. XiBao Yu FenZi MianYiXue ZaZhi. 2001;17:549-551. |
| 22. | Ding J, Liu J, Xue CF, Li YH, Gong WD. Construction and expression of prokaryotic expression vector for pTAT-HBV targeted ribonuclease fusion protein. XiBao Yu FenZi MianYiXue ZaZhi. 2003;19:49-51. [PubMed] |
| 23. | Gong WD, Liu J, Ding J, Zhao Y, Li YH, Xue CF. Optimizing HBV targeted ribonuclease by introduction of linker. Disi Junyi Daxue Xuebao. 2003;24:1011-1013. |
| 24. | Zhao Y, Liu J, Gong WD, Li YH, Ding J, Xue CF. Expression of fusion protein gene of HBV core protein and human eosinophil derived neurotoxin mediated by recombinant adenovirus in mouse liver. Disi Junyi Daxue Xuebao. 2004;25:1073-1076. |
| 25. | Zhao Y, Gong WD, Liu J, Li YH, Ding J, Xue CF. Construction of the HBV-TR recombinant adenovirus vector. Disi Junyi Daxue Xuebao. 2004;25:321-324. |
| 26. | Raj V. Treatment of hepatitis B. Clin Cornerstone. 2001;3:24-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Sprinzl MF, Oberwinkler H, Schaller H, Protzer U. Transfer of hepatitis B virus genome by adenovirus vectors into cultured cells and mice: crossing the species barrier. J Virol. 2001;75:5108-5118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 124] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Nilsson M, Ljungberg J, Richter J, Kiefer T, Magnusson M, Lieber A, Widegren B, Karlsson S, Fan X. Development of an adenoviral vector system with adenovirus serotype 35 tropism; efficient transient gene transfer into primary malignant hematopoietic cells. J Gene Med. 2004;6:631-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Zeng M, Smith SK, Siegel F, Shi Z, Van Kampen KR, Elmets CA, Tang DC. AdEasy system made easier by selecting the viral backbone plasmid preceding homologous recombination. Biotechniques. 2001;31:260-262. [PubMed] |
Science Editor Li WZ Language Editor Elsevier HK