Published online May 7, 2005. doi: 10.3748/wjg.v11.i17.2552
Revised: April 23, 2004
Accepted: July 27, 2004
Published online: May 7, 2005
AIM: Because of a major resistance to chemotherapy, prognosis of hepatocellular carcinoma (HCC) is still poor. New treatments are required and gene therapy may be an option. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) induces apoptosis in multiple malignant tumors, and using adenoviral vectors has shown a targeted tumor-specific therapy. However, repeated administration of adenoviral vectors can lead to cell resistance, which may be caused by the initial coxsackie-adenovirus receptor (CAR). One technique to overcome resistance is the use of modified adenoviral vectors containing an Arg-Gly-Asp (RGD) sequence. In this study we constructed an adenoviral vector (designated Ad/TRAIL-F/RGD) with RGD-modified fibers, expressing the TRAIL gene from the human telomerase reverse transcriptase (hTERT) promoter, and evaluated its antitumor activity in HCC cell lines.
METHODS: To investigate the effects of Ad/TRAIL-F/RGD in human HCC cell lines Hep G2 and Hep 3b, cells were infected with Ad/CMV-GFP (vector control), Ad/gTRAIL (positive control), and Ad/TRAIL-F/RGD. Phosphate-buffered saline (PBS) was used as control. Cell viability was determined by proliferation assay (XTT), and apoptosis induction by fluorescence activated cell sorting (FACS).
RESULTS: Cells treated with Ad/TRAIL-F/RGD and Ad/gTRAIL showed a significantly reduced cell viability in comparison to PBS and Ad/CMV-GFP treatment in both cell lines. Whereas, treatment with PBS and Ad/CMV-GFP had no cell-killing effect. The reduced cell viability was caused by induction of apoptosis as shown by FACS analysis. The amount of apoptotic cells was similar after incubation with Ad/gTRAIL and Ad/TRAIL-F/RGD.
CONCLUSION: The new RGD modified vector Ad/TRAIL-F/RGD could become a potent therapeutic agent for the treatment of HCC, adenovirus resistant tumors, and CAR low or negative cancer cells.
- Citation: Jacob D, Schumacher G, Bahra M, Davis J, Zhu HB, Zhang LD, Teraishi F, Neuhaus P, Fang BL. Fiber-modified adenoviral vector expressing the tumor necrosis factor-related apoptosis-inducing ligand gene from the human telomerase reverse transcriptase promoter induces apoptosis in human hepatocellular carcinoma cells. World J Gastroenterol 2005; 11(17): 2552-2556
- URL: https://www.wjgnet.com/1007-9327/full/v11/i17/2552.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i17.2552
Hepatocellular carcinoma (HCC) represents up to 80% of all primary malignant liver tumors with an increasing incidence worldwide[1]. The most common risk factors for this disease are liver cirrhosis, chronic infections with hepatitis B and hepatitis C viruses, and exposure to aflatoxins[2]. Surgical resection and liver transplantation are therapies of choice, with potential cure, but at the time of diagnosis, about 80% of no curative therapy is possible. Local tumor ablations such as radiofrequency ablation, and laser-induced thermoablation may be a good alternative, but the indication is restricted only to selected patients with small tumors. Chemoembolization has no effect on long-term survival, and no promising radio/chemotherapy is available[3].
Because of a poor outcome in non-resectable HCCs, new treatment strategies are of high clinical interest, and gene therapy may be an option. One reason for focus on gene therapy is the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), which is a death-inducing ligand belonging to the tumor necrosis factor (TNF)-cytokine superfamily.
It has recently been demonstrated that the direct transfer of the TRAIL gene via adenoviral vectors into a variety of human cancer cells could induce strong apoptosis in vitro and suppress tumor growth significantly in vivo[4-6].
However, repeated application of apoptosis-inducing adenoviral vectors can result in the selection and expansion of resistant cells, and resistance to adenoviral infections is presumably caused by a low expression of the initial binding receptor, the coxsackie-adenovirus receptor (CAR) or integrins, such as αvβ3, αvβ5, or αvβ1[7-9]. In addition, reduced expression of CAR has also been reported in primary tumors[10], suggesting that overcoming resistance to adenoviruses in cancer cells is important for the future success of adenovirus mediated cancer gene therapy in patients.
Adenoviral capsid proteins can be modified to retarget adenovectors to CAR-independent binding molecules. One technique is to modify the adenoviral vector by incorporating the integrin-binding motif RGD sequence into the HI loop of the adenoviral fiber proteins.
Therefore, we constructed an adenoviral vector broadly applicable to cancer therapy, designated as Ad/TRAIL-F/RGD. This new vector was modified with the RGD sequence in the HI loop of fibers and expressed the TRAIL gene from the hTERT promoter via GAL4 gene regulatory components that could augment transgene expression from the tumor-specific promoter without losing target specificity[11].
We evaluated the efficiency of Ad/TRAIL-F/RGD in comparison to our previously constructed adenoviral vector Ad/gTRAIL in vitro in two HCC cell lines. Our results showed a strongly reduced viability and apoptotic effect of Ad/TRAIL-F/RGD in both cell lines.
The adenoviral vectors Ad/CMV-GFP and Ad/gTRAIL were described previously[5]. Ad/TRAIL-F/RGD was constructed by co-transfecting 293 cells with a shuttle plasmid expressing a full-length human TRAIL coding sequencing from the hTERT promoter and a 30 kb ClaI fragment from Ad/LacZ-F/RGD as described previously[12]. The expansion, purification, titration, and quality analyses of all these vectors were performed at the vector core facility at The University of Texas MD Anderson Cancer Center as previously described[13]. All viral preparations were found to be free of the E1+ adenovirus using polymerase chain reaction (PCR)[13] and to be free of endotoxins using a Limulus amebocyte lysate endotoxin detection kit (BioWhittaker, Walkersville, MD). The titer used in this study was determined by the absorbency of the dissociated viruses at A260 nm (one A260 nm unit = 1012 viral particles [VP]/mL), and the titers determined with a plaque assay were used to determine additive information. Particle: infectious unit ratios were usually between 30:1 and 100:1. Thus, the multiplicity of infection (MOI) of 1000 VP was equivalent to a multiplicity of infection of 10-30 infectious units. Unless otherwise specified, Ad/CMV-GFP was used as the vector control and phosphate-buffered saline (PBS) as a control.
Human HCC cell lines Hep G2 and Hep 3b were purchased from the American Type Culture Collection (ATCC) (Rockville, MD). Both cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 100 mL/L heat-inactivated fetal bovine serum (CFBS), 1% glutamine, and 1% penicillin and streptomycin (Gibco-BRL, Life Techologies, Inc., Grand Island, NY). All cells were cultured at 37 °C in a humidified incubator containing 50 mL/L CO2.
Cell viability was determined using a 3-bis-(2-methoxy-4-nitro-5 sulfenyl)-(2H)-tetrazolium-5-carboxanilide (XTT) assay (Cell Proliferation Kit II, Roche Molecular Biochemicals, Indianapolis, IN, USA), as described previously[13]. Briefly, 1×104 cells/plate were seeded onto a 96-well plate, and after 24 h Ad/CMV-GFP, Ad/gTRAIL, or Ad/TRAIL-F/RGD were added to each well with a multiplicity of infection (MOI) of 2000 VP/cell. PBS was used as a control. Cells were incubated at 37 °C in a humidified atmosphere containing 50 mL/L CO2. A XTT assay was performed for the following 5 d after the treatment. Each experiment was performed in quadruplicate and repeated at least twice. Data are expressed as mean±SD.
Fluorescence-activated cell sorting (FACS) was performed to determine in vitro apoptosis induction. The Hep G2 and Hep3b cells were plated onto 100 mm plates at a density of 1×106 cells/plate a day before treatment. Cells were infected with Ad/CMV-GFP, Ad/gTRAIL, or Ad/TRAIL-F/RGD using a MOI of 1000 VP/cell. PBS was used as a control. After incubation for 48 h, both adherent and floating cells were harvested (adherent by trypsinization) and washed with PBS. The cells were then fixed with 70% ethanol overnight and stained with propidium iodide (PI) (1 mL of PI, 10 µL of RNase, 9 mL of PBS, PI: 50 µg/mL) before analysis. This procedure was done using flow cytometry, the sub-G0/G1 cellular DNA content was measured using Cell Quest software (Becton-Dickinson, San Jose, CA, USA).
Statistical differences among the treatment groups were assessed by ANOVA using the Statistica software program (StatSoft, Tulsa, OK). P<0.05 was considered statistically significant.
Ad/TRAIL-F/RGD had the same bicistronic expression cassette as Ad/gTRAIL[5] except that wild-type TRAIL cDNA was used instead of the GFP/TRAIL fusion construct described in Ad/gTRAIL. In addition, Ad/TRAIL-F/RGD contained an insertion of the CDCRGDCFC sequence in the HI loop of fibers and a deletion in the E3 region from 28599 to 30469 bp. The sequences of the E3 region, the fiber region, human TRAIL cDNA, and the GAL4/VP16 fusion gene in the E1 region in Ad/TRAIL-F/RGD were verified by automatic DNA sequencing using purified viral DNAs as templates.
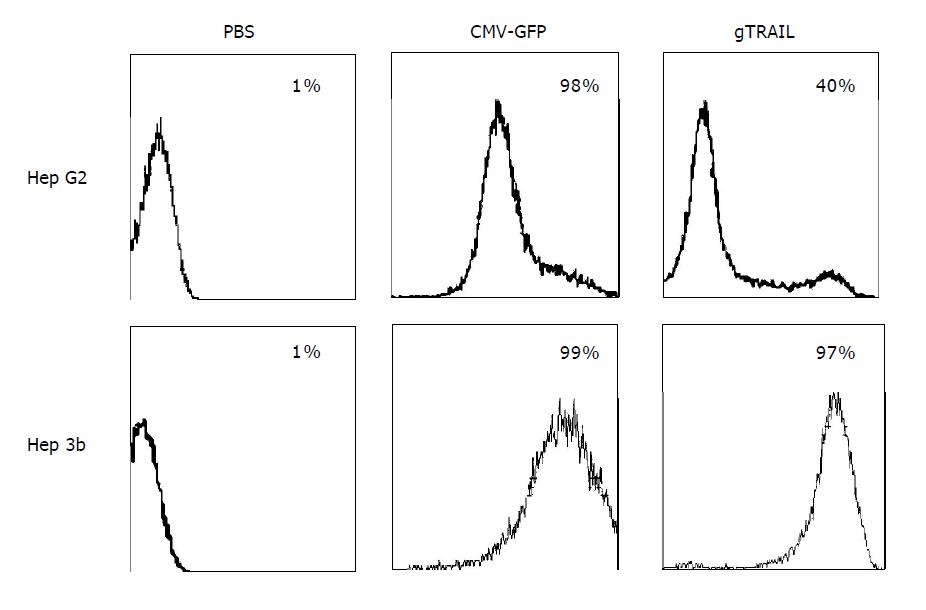
We transfected both cell lines with Ad/CMV-GFP and Ad/gTRAIL for evaluation if the cell lines were capable of transfecting adenoviruses. PBS was used as a control. Cells were transfected with a MOI of 1000 VP/cell and the GFP expression was measured after 48 h of treatment by FACS analysis. Hep G2 and Hep 3b showed a strong transfection rate for Ad/CMV-GFP between 98% and 99% and for Ad/gTRAIL between 40% and 97% (Figure 1).
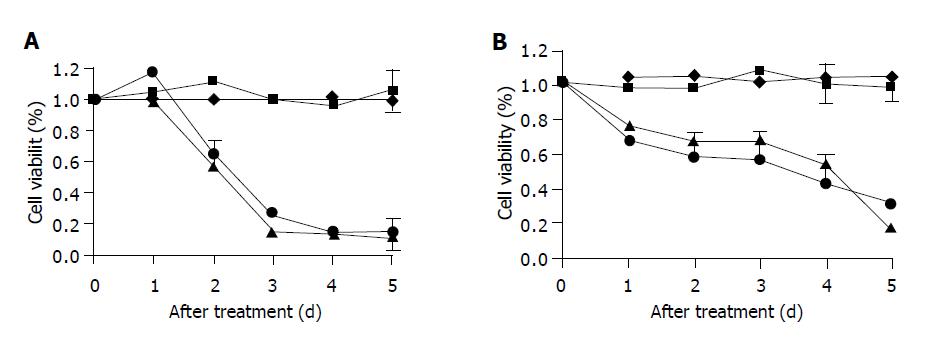
We examined the effects of Ad/TRAIL-F/RGD on HCC cell lines Hep G2 (Figure 2A) and Hep 3b (Figure 2B). Both cell lines were sensitive to Ad/TRAIL-F/RGD and Ad/gTRAIL and showed a significantly reduced viability compared to cells treated with PBS or Ad/CMV-GFP, after 5 d of treatment in all groups (P<0.05). The viability reducing effect was not significant between Ad/gTRAIL and Ad/TRAIL-F/RGD.
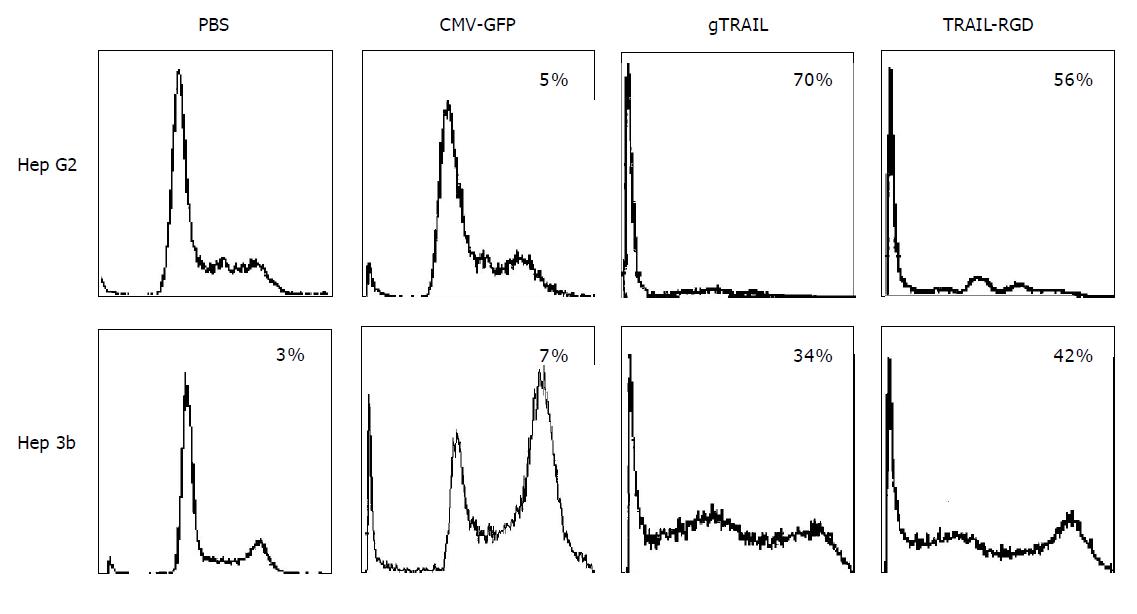
To further evaluate the apoptosis caused by Ad/TRAIL-F/RGD and Ad/gTRAIL, we quantified the sub-G1 population in both cancer cell lines by FACS analysis. The MOI for the adenoviral treatment was set at 1000 VP/cell. Ad/gTRAIL and Ad/TRAIL-F/RGD dramatically increased the percentage of apoptotic cells in comparison to cells treated with PBS, or Ad/CMV-GFP. The apoptotic effect between Ad/gTRAIL and Ad/TRAIL-F/RGD was almost equal and cells treated with PBS or Ad/CMV-GFP had only background levels of apoptosis (Figure 3).
Gene therapy may be an alternative approach, using the proapoptotic tumor necrosis factor-related apoptosis-inducing ligand (TRAIL).
TRAIL belongs to the TNF-α cytokine super-family, including TNF-α, FasL/CD95/Apo1L, TRAIL/Apo2L, and TWEAK/DR3L/Apo3L[15]. These ligands are type-II transmembrane proteins and membrane-bound TRAIL as recombinant soluble TRAIL could rapidly induce apoptosis in various cancer cells by interaction with DR4/TRAIL-R1 or DR5/TRAIL-2 death receptors[15]. It has recently been reported that expression of the TRAIL or TRAIL/GFP fusion gene could induce apoptosis in a wide variety of cancer cells[5,15-17,25,26]. Moreover, the direct transfer of the TRAIL gene resulted in an apoptotic bystander effect including non-transfected neighboring cancer cells[4,15]. However, one major concern of proapoptotic gene therapy is its systemic toxicity. Using tumor-specific promoters with targeted gene expression can avoid this effect. For this reason, we used the human telomerase reverse transcriptase (hTERT) promoter, whose gene was active in 85% of human cancer cells, but inactive in most somatic cells[5]. But a limitation of tissue- or cell-type-specific promoters to target transgene expression was hampered by their weak transcriptional activity[11]. A solution for this problem is the GAL4 gene regulatory system, wherein a weak tissue-specific promoter could drive expression of the GAL4/VP16 fusion protein (GV 16), which in turn could transactivate a minimal synthetic promoter, GAL4/TATA (GT), which upstreams the TRAIL gene[11]. We recently constructed an adenoviral vector, which could combine these qualities, expressing the GFP/TRAIL fusion gene driven by the hTERT promoter via GAL4 gene regulatory components (designated as Ad/gTRAIL). This vector prevented transgene expression and toxicity in primary human hepatocytes[5], but led to apoptosis in multiple human tumor cell lines[4-6].
Nevertheless, repeated administration of adenovectors, as a gene transport delivery device, could result in cell resistance to adenovirus binding mechanism, especially the initial CAR[7,9,18]. Moreover, reduced expression of CAR has also been reported in primary tumors[10]. Thence, new techniques to increase the transfection efficiency are desirable. A simple solution could be the increase of the viral titer, but it would lead to more systemic toxicity in animal experiments or clinical trials. An alternative way is to retarget the adenoviral capsid protein by modifying adenoviral vectors to CAR-independent binding molecules. One technique is the incorporating of the integrin-binding motif RGD sequence into the HI loop of the adenoviral fiber protein.
Recently published reports showed a significantly increased transduction efficiency in oral squamous cell carcinoma[19] and cervix carcinoma cells[20], ovarian cancer cells[21], melanoma cells[22], and glioma cells[23].
Therefore, modified vectors containing polylysine or an RGD sequence might have a broader application than conventional adenovectors in targeted cancer gene therapy[8,9], especially for in vivo experiments. These vectors could overcome resistance to adenoviral treatment, and increase the transfection efficiency in cells with a low CAR expression. Consequently, we constructed an adenoviral vector expressing the wild-type TRAIL gene from the hTERT promoter and contained an RGD sequence in the HI loop of its fiber protein (Ad/TRAIL-F/RGD), which would have a broader application than the vectors previously reported. We compared the new vector with our previously constructed adenoviral vector Ad/gTRAIL as a positive control, which showed a strong apoptotic effect on a variety of human tumor cells[4-6].
The in vitro experiments of our study showed that Ad/TRAIL-F/RGD significantly reduced the cell viability in Hep G2 and Hep 3b cells in comparison to Ad/CMV-GFP and PBS treated cells. FACS analysis concerned these results. Both experiments showed no significant difference between Ad/gTRAIL and the new Ad/TRAIL-F/RGD in both cell lines. These results confirmed that the modification of our previously demonstrated vector Ad/gTRAIL did not decrease the proapototic efficiency in vitro. It would be interesting to compare both vectors in vivo in tumor models with CAR low or negative cell lines, but so far no tumor model with these criteria has been established. However, we have previously reported that the pancreas carcinoma cell line MIA PaCa-2 has a low CAR expression[8] and might be an option for a tumor model, because of its tumorigenicity in nude mice. Furthermore, it has recently been found that some chemotherapeutic agents have a synergistic effect in combination with adenovirus mediated TRAIL synthesis, even on chemoresistant cancer cells[17,24]. These results and the increased transfection rate by fiber modified adenoviruses in CAR low cell lines may reduce the virus particles for clinical studies, which seems to be an effective treatment without side effects due to high adenovirus titers.
We thank Wang XL for editorial review and Henry Peng in the Keck Vector Core for adenovirus propagation and quality control.
| 1. | Ince N, Wands JR. The increasing incidence of hepatocellular carcinoma. N Engl J Med. 1999;340:798-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 110] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Wands JR, Blum HE. Primary hepatocellular carcinoma. N Engl J Med. 1991;325:729-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 78] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Simonetti RG, Liberati A, Angiolini C, Pagliaro L. Treatment of hepatocellular carcinoma: a systematic review of randomized controlled trials. Ann Oncol. 1997;8:117-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 280] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 4. | Kagawa S, He C, Gu J, Koch P, Rha SJ, Roth JA, Curley SA, Stephens LC, Fang B. Antitumor activity and bystander effects of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene. Cancer Res. 2001;61:3330-3338. [PubMed] |
| 5. | Lin T, Gu J, Zhang L, Huang X, Stephens LC, Curley SA, Fang B. Targeted expression of green fluorescent protein/tumor necrosis factor-related apoptosis-inducing ligand fusion protein from human telomerase reverse transcriptase promoter elicits antitumor activity without toxic effects on primary human hepatocytes. Cancer Res. 2002;62:3620-3625. [PubMed] |
| 6. | Voelkel-Johnson C, King DL, Norris JS. Resistance of prostate cancer cells to soluble TNF-related apoptosis-inducing ligand (TRAIL/Apo2L) can be overcome by doxorubicin or adenoviral delivery of full-length TRAIL. Cancer Gene Ther. 2002;9:164-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Bergelson JM, Krithivas A, Celi L, Droguett G, Horwitz MS, Wickham T, Crowell RL, Finberg RW. The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J Virol. 1998;72:415-419. [PubMed] |
| 8. | Pearson AS, Koch PE, Atkinson N, Xiong M, Finberg RW, Roth JA, Fang B. Factors limiting adenovirus-mediated gene transfer into human lung and pancreatic cancer cell lines. Clin Cancer Res. 1999;5:4208-4213. [PubMed] |
| 9. | Zhang L, Gu J, Lin T, Huang X, Roth JA, Fang B. Mechanisms involved in development of resistance to adenovirus-mediated proapoptotic gene therapy in DLD1 human colon cancer cell line. Gene Ther. 2002;9:1262-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Jee YS, Lee SG, Lee JC, Kim MJ, Lee JJ, Kim DY, Park SW, Sung MW, Heo DS. Reduced expression of coxsackievirus and adenovirus receptor (CAR) in tumor tissue compared to normal epithelium in head and neck squamous cell carcinoma patients. Anticancer Res. 2002;22:2629-2634. [PubMed] |
| 11. | Koch PE, Guo ZS, Kagawa S, Gu J, Roth JA, Fang B. Augmenting transgene expression from carcinoembryonic antigen (CEA) promoter via a GAL4 gene regulatory system. Mol Ther. 2001;3:278-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Fang B, Ji L, Bouvet M, Roth JA. Evaluation of GAL4/TATA in vivo. Induction of transgene expression by adenovirally mediated gene codelivery. J Biol Chem. 1998;273:4972-4975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Gu J, Kagawa S, Takakura M, Kyo S, Inoue M, Roth JA, Fang B. Tumor-specific transgene expression from the human telomerase reverse transcriptase promoter enables targeting of the therapeutic effects of the Bax gene to cancers. Cancer Res. 2000;60:5359-5364. [PubMed] |
| 14. | Bruix J, Llovet JM. Locoregional treatments for hepatocellular carcinoma. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:611-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Lin T, Huang X, Gu J, Zhang L, Roth JA, Xiong M, Curley SA, Yu Y, Hunt KK, Fang B. Long-term tumor-free survival from treatment with the GFP-TRAIL fusion gene expressed from the hTERT promoter in breast cancer cells. Oncogene. 2002;21:8020-8028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Griffith TS, Anderson RD, Davidson BL, Williams RD, Ratliff TL. Adenoviral-mediated transfer of the TNF-related apoptosis-inducing ligand/Apo-2 ligand gene induces tumor cell apoptosis. J Immunol. 2000;165:2886-2894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 145] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Lin T, Zhang L, Davis J, Gu J, Nishizaki M, Ji L, Roth JA, Xiong M, Fang B. Combination of TRAIL gene therapy and chemotherapy enhances antitumor and antimetastasis effects in chemosensitive and chemoresistant breast cancers. Mol Ther. 2003;8:441-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Miller CR, Buchsbaum DJ, Reynolds PN, Douglas JT, Gillespie GY, Mayo MS, Raben D, Curiel DT. Differential susceptibility of primary and established human glioma cells to adenovirus infection: targeting via the epidermal growth factor receptor achieves fiber receptor-independent gene transfer. Cancer Res. 1998;58:5738-5748. [PubMed] |
| 19. | Dehari H, Ito Y, Nakamura T, Kobune M, Sasaki K, Yonekura N, Kohama G, Hamada H. Enhanced antitumor effect of RGD fiber-modified adenovirus for gene therapy of oral cancer. Cancer Gene Ther. 2003;10:75-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Wu H, Seki T, Dmitriev I, Uil T, Kashentseva E, Han T, Curiel DT. Double modification of adenovirus fiber with RGD and polylysine motifs improves coxsackievirus-adenovirus receptor-independent gene transfer efficiency. Hum Gene Ther. 2002;13:1647-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 109] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Kanerva A, Wang M, Bauerschmitz GJ, Lam JT, Desmond RA, Bhoola SM, Barnes MN, Alvarez RD, Siegal GP, Curiel DT. Gene transfer to ovarian cancer versus normal tissues with fiber-modified adenoviruses. Mol Ther. 2002;5:695-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 141] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 22. | Nakamura T, Sato K, Hamada H. Effective gene transfer to human melanomas via integrin-targeted adenoviral vectors. Hum Gene Ther. 2002;13:613-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Koizumi N, Mizuguchi H, Hosono T, Ishii-Watabe A, Uchida E, Utoguchi N, Watanabe Y, Hayakawa T. Efficient gene transfer by fiber-mutant adenoviral vectors containing RGD peptide. Biochim Biophys Acta. 2001;1568:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Uchida H, Shinoura N, Kitayama J, Watanabe T, Nagawa H, Hamada H. 5-Fluorouracil efficiently enhanced apoptosis induced by adenovirus-mediated transfer of caspase-8 in DLD-1 colon cancer cells. J Gene Med. 2003;5:287-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Jacob D, Davis J, Zhu H, Zhang L, Teraishi F, Wu S, Marini FC, Fang B. Suppressing orthotopic pancreatic tumor growth with a fiber-modified adenovector expressing the TRAIL gene from the human telomerase reverse transcriptase promoter. Clin Cancer Res. 2004;10:3535-3541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Huang X, Lin T, Gu J, Zhang L, Roth JA, Stephens LC, Yu Y, Liu J, Fang B. Combined TRAIL and Bax gene therapy prolonged survival in mice with ovarian cancer xenograft. Gene Ther. 2002;9:1379-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |