Published online Apr 28, 2005. doi: 10.3748/wjg.v11.i16.2497
Revised: March 21, 2004
Accepted: April 29, 2004
Published online: April 28, 2005
AIM: To study the inhibitory effects of siRNAs targeting different hTERT sequences and to screen the effective siRNA sequence.
METHODS: Five double-stranded siRNAs targeting coding and non-coding regions of hTERT gene were designed and synthesized by T7 transcription system in vitro. siRNA4 sequence was screened by full length gene targeting technique and the rest of the siRNA sequences were selected randomly. After being purified by ethanol precipitation, the siRNAs were transfected to the human hepatocellular carcinoma cell (HepG2) by LipofectamineTM2000. At 48-72 h after siRNAs transfection, MTT assay, RT-PCR and Western-blot were applied to evaluate the effects of siRNAs on cell growth, mRNA and protein expression level of hTERT gene, respectively.
RESULTS: Compared to the control cells, the cells treated with the five double-stranded siRNAs exhibited different degrees of inhibition of cell proliferation in a dose-dependent manner. siRNA2 and siRNA4, exhibited obvious effects of inhibiting hTERT mRNA and protein expression in HepG2 cells.
CONCLUSION: siRNAs targeting different hTERT sequences have significantly various inhibitory effects on hTERT gene expression. The siRNA sequence screened by full length gene targeting technique has comparable inhibitory effect with the rest siRNA sequences screened by random selection, suggesting that siRNAs and antisense oligonucleic acids may have the same effective target sites. Compared with chemical synthesis method, synthesizing double-stranded siRNA by T7 transcription system in vitro is a rapid, simple, and inexpensive method suitable for screening high-effect siRNA targeting site for specific gene.
- Citation: Xia Y, Lin RX, Zheng SJ, Yang Y, Bo XC, Zhu DY, Wang SQ. Effective siRNA targets screening for human telomerase reverse transcriptase. World J Gastroenterol 2005; 11(16): 2497-2501
- URL: https://www.wjgnet.com/1007-9327/full/v11/i16/2497.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i16.2497
Telomerase enzyme complex have two major subunits contributing to enzymatic activity: a structural RNA component (human telomerase RNA, hTER) that contains a template region that binds the TTAGGG repeats in telomerase and a catalytic subunit with reverse transcriptase activity (human telomerase reverse transcriptase, hTERT). Expression of hTERT is almost exclusively limited to cancer cells and recent research indicated that hTERT expression is a rate-limiting step in telomerase activity and carcinogenesis[1]. Inhibition of hTERT activity has potential significance in gene function research and cancer gene therapy.
RNA interference (RNAi) is a sequence-specific post-transcriptional gene silencing mechanism, which is triggered by double-stranded RNA (dsRNA), causing degradation of mRNAs homologous in sequence to the dsRNA and inhibiting specific gene expression effectively[2]. As for antisense DNA and ribozymes, various siRNAs directed at different sites of target gene exhibit obviously different suppression effects. Thus, siRNA faces the same challenges that confront other nucleic acid-based gene inactivation strategies: site selection[3]. In the present study, five siRNA sequences targeting at different sites of hTERT gene were designed by the method of random selection and full length gene targeting technique based on RNase digestion sensitivity. All of them were synthesized by T7 RNA polymerase in vitro and the inhibitory effects were evaluated on cell proliferation, hTERT mRNA and protein expression, respectively.
The principle of random selection of siRNA target sites was described previously[4]. In the totally five siRNA sequences, siRNA4 was selected by full length gene targeting technique established by our laboratory, the rest were selected by random selection method. All siRNA sequences were done blast-research in GenBank to confirm that only hTERT gene was targeted. The information of the five siRNAs is summarized in Table 1.
| Number | Position in hTERT mRNA | Sense sequence(5’-3’) | GC content (%) |
| 1 | 2319-2329 (coding region) | AAGGC ACT GTT CAG CGT GCTC | 57 |
| 2 | 2653-2673 (coding region) | AAGGC CTT CAA GAG CCA CGTC | 57 |
| 3 | 1801-1821 (coding region) | AAGGT GCA AAG CAT TGG AATC | 38 |
| 4 | 3652-3672 (non-coding region) | AAGGG CTG AGT GTC CAG CACA | 57 |
| 5 | 3865-3885 (non-coding region) | AAGGA CCC TGG GAG CTC TGGG | 67 |
For in vitro transcription to produce 21-nt siRNA, four strands of 43-nt DNA template oligonucleotides were synthesized as:
P1: 5’ T7 promoter Sense sequence (19 nt of AA down-stream) TT 3’
P2: 5’ T7 promoter Antisense sequence TT 3’
P3 was complementary with P1; P4 was complementary with P2. T7 promoter sequence was: 5’-GGTAATACGAC-TCACTATAGGG-3’. The underlined G was the initiating site of transcription. The 43nt DNA oligomers were synthesized by an applied biosystems 391 DNA synthesizer and purified by PAGE.
DNA template strands P1 and P3, were mixed in equimolar amounts, heated for 5 min at 95 °C, then gradually cooled down to room temperature in annealing buffer to form the double-stranded DNA S1. P2 and P4 were treated the same way to form the double-stranded DNA S2. Transcription in vitro was carried out in two separate tubes by using the RiboMaxTM Large Scale RNA Production System-T7 Kit (Promega) according to the manufacturer’s instructions to obtain two single-stranded RNAs. The two complementary single-stranded RNAs were mixed and incubated at 37 °C overnight to form double-stranded RNA. Then the product was treated with DNase and single-stranded specific RNase T1 for 30 min at 37 °C to digest DNA template, unpaired single-stranded RNA and the 5’ overhung GGG in double-stranded RNA was cleaved. The double-stranded RNA was then purified by ethanol precipitation and resolved with Nuclease-Free water. The product was the siRNA for transfection with 3’ end overhung UU bases. RNAs were quantified by DU640 Nucleic Acid Analyzer (Beckman Coulter) and stored at -20 °C.
The human hepatocellular carcinoma cell HepG2 (American Type Culture Collection, Rockville, MD) were maintained in DMEM medium containing 10% heat-inactivated fetal calf serum (Gibco Brl) and incubated at 37 °C, 5% CO2 atmosphere in a humidified incubator. Cells were regularly passaged to maintain exponential growth.
The day before transfection, cells were seeded at a density of 5×103 cells/well in 96-well flat-bottomed plates (0.1 mL/well) and cultured for about 24 h at 37 °C, 5% CO2 atmosphere. When the cells reached 40-50% confluence, they were transfected with siRNAs complexed with LipofectamineTM 2000 (Invitrogen) according to manufacturer’s instructions in triplicate for each concentration. After an incubation for 48 h at 37 °C, 20 μL MTS agent (Promega) was added to each well followed by another 90 min incubation at 37 °C. Absorption was measured at 490-nm (Victor 1420 Multilabel Counter, Wallac) and inhibitory rates on cell proliferation was evaluated using the following formula:
Inhibitory rate=(Acontrol-Asample)/(Acontrol-Ablank)×100%
Acontrol: Absorption of cells treated with LipofectamineTM2000 only; Asample: Absorption of cell treated with siRNA and LipofectamineTM2000; Ablank: Absorption of DMEM.
Totally 1.0×105 cells were seeded in a six-well plate. After 24 h incubation, cell confluence was about 50%. siRNAs were mixed with LipofectamineTM2000 and transfected to HepG2 cells with the final concentration 100 nmol/L. After an incubation for 48 h at 37 °C, total RNA was isolated by TRIzol (Invitrogen) using a single-step phenol-extraction method. The cDNA strand was synthesized from 2 μg of RNA by using Superscriptase II (Invitrogen) according to the manufacturer’s instructions. PCR primers were as follows: hTERT, 5’-TCTACCGGAAGAGTGTCTGGA-GCAA-3’ (forward) and 5’-GCTCCCACGACGTAGTC-CATGTTCA-3’ (reversed); amplicon, 202 bp; β2-microglobulin 5’ TTCAGGTTTACTCACGTCATCC-3’ (forward), and 5’-CCAAATGCGGCATCTTCAAACCC-3’ (reversed); amplicon, 317 bp. PCR reaction for hTERT and β2-microglobulin was performed according to a method described earlier. PCR products were run on a 2.0% agarose gel and visualized by ethidium bromide staining. The intensities of DNA bands were measured by scanning the gel with Gel Doc 1000 (Bio-Rad). Inhibition of hTERT mRNA was calculated by relative intensity ratio hTERT/β2-microglobulin according to the following formula:
Inhibitory rate (%) =(1-Asample×A0control/Acontrol×A0sample)×100
Asample: the intensity of hTERT PCR product in cells treated with 100 nM siRNA, A0control: the intensity of β2-microglobulin product in cells treated with LipofectamineTM2000 alone, Acontrol: the intensity of β2-microglobulin product in cells treated with 100 nmol/L siRNA, A0sample: the intensity of hTERT PCR product in cells treated with LipofectamineTM2000 alone.
Cells treated with 100 nmol/L siRNAs were harvested 72 h after transfection and lysed in a lysis buffer (RIPA-PICT, Roche) for 30 min on ice. A total of 40 μg of protein extracted from each transfected cell population was separated on 8% acrylamide gels using standard SDS-PAGE techniques and then transferred to Hybond-polyvinylidene difluoride membrane (Amersham Biosciences). The membrane was blocked overnight at 4 °C and then probed with 1:2000 diluted anti-hTERT specific antibody (Alpha Diagnostic) for 1 h at room temperature. The membrane was washed thrice for 15 min each with TBST buffer, incubated with 1:2000 diluted HRP-conjugated secondary antibody (Sigma) for 1 h at room temperature, washed four times for 15 min each with TBST buffer. Finally, membrane was incubated with ECL-PLUS reagent (Amersham Biosciences) for 5 min at room temperature to develop the bands, which were scanned by Typhoon 9410 Variable Mode Imager (Amersham Pharmacia). β-Actin was used as control for loading equal amount of protein in each gel. The inhibition rate was calculated in the same manner as RT-PCR.
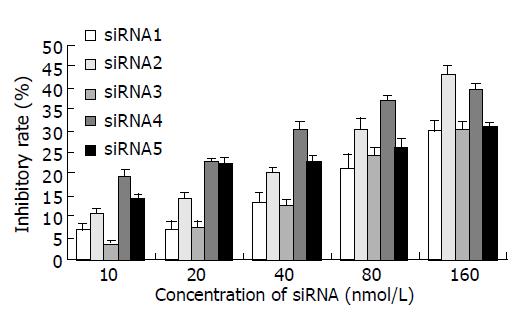
All of the five siRNAs showed inhibitory effects on HepG2 cell growth in a dose-dependent manner (Figure 1). siRNA2 had the highest inhibitory effect at a dose of 160 nmol/L (43.1%) and siRNA4 had better inhibitory effects at doses 10-80 nmol/L compared with the other siRNAs, it was very close to the inhibitory rate of siRNA2 at a dose 160 nmol/L (39.6%). These results suggested that siRNA targeting hTERT gene could effectively suppress HepG2 cell growth.
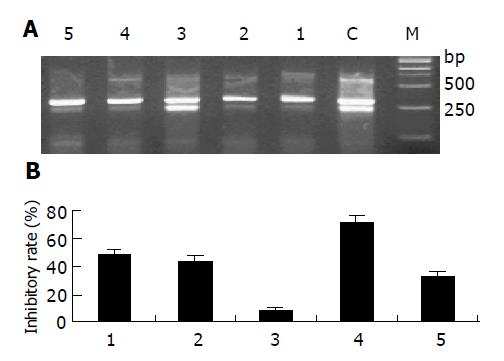
In order to detect whether siRNA could inhibit hTERT gene expression, the mRNA level of hTERT was determined by semi-quantitative RT-PCR. A 202-bp DNA fragment for hTERT gene and a 317-bp DNA fragment for β2-microglobulin gene were amplified by RT-PCR with specific primers, respectively. The result of RT-PCR showed that hTERT mRNA expression level was decreased after 48-h treatment with 100 nmol/L siRNAs when compared to the control cell except siRNA3 (Figure 2A). Normalized to the levels of β2-microglobulin, the relative inhibitory rates of siRNA1-siRNA5 were 48.3%, 43.7%, 8.3%, 70.7%, 32.7%, respectively (Figure 2B).
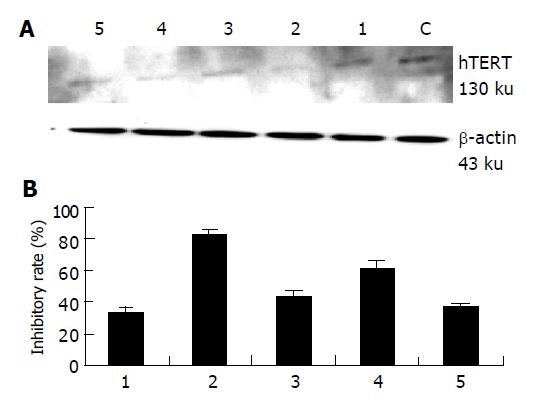
Western-blot analysis was performed to determine the effects of siRNAs treatment on hTERT protein level in HepG2 cells. A 130 ku band for hTERT protein was scanned and normalized to β-actin protein (Figure 3A). The relative inhibitory percentage of hTERT protein expression in the cells treated with 100 nmol/L siRNA1-siRNA5 was 33.0%, 82.5%, 43.0%, 61.5%, 36.5%, respectively (Figure 3B).
Short interfering RNAs (siRNAs) are powerful sequence-specific reagents designed to knockdown the expression of target genes in cultured mammalian cells through a process known as RNA interference (RNAi). Although the mechanism underlying RNAi activity has not been completely elucidated, RNAi has already become a powerful reverse genetic method for suppressing the expression of a target gene. Recent studies have demonstrated that 21 nt-siRNA duplexes are long enough to induce gene-specific suppression, but short enough to evade the host interferon response in cultured mammalian cells[5].The duplexes of 21 nt RNAs with symmetric 2-nt 3’ overhangs are the most efficient mediators of mRNA degradation[6]. Especially, 2-nt 3’ overhangs in antisense strand of siRNA are crucial for inducing RNAi in mammalian cells[7]. In the present study, four strands of 43 nt DNA template oligonucleotides were designed for synthesizing two single-stranded RNAs in vitro by using T7 RNA polymerase. The two complementary single-stranded RNAs could form 24 nt duplexes RNA with symmetric UU 3’ overhangs and GGG 5’ overhangs through Watson-Crik hybridization. GGG 5’ overhangs in RNA duplexes were cleaved by single-stranded specific RNase T1 and the product was 21 nt siRNA for transfection with symmetric UU 3’ overhangs.
In mammalian cells, it has recently been reported that siRNA efficacy is highly dependent upon target position, that is, the secondary structure of target RNA is an important determinant of activity for siRNA[8]. Currently selection of the targeted region is largely empirical. At the moment, there are no reliable ways to predict or identify the “ideal” sequence for an siRNA. Generally, the mRNA sequences of the desired gene were scanned randomly for AA sequences, then the AA and the downstream 19 nt were recorded and compared to an appropriate genome database to eliminate any with significant homology to other genes. Those sequences are the potential siRNA target sites[6]. Since siRNA, like antisense oligonucleotides, through an antisense mechanism results in loss of target RNA, it is reasonable to deduce that the degree of RNase H sensitivity of a given probe reflects the accessibility of the chosen site and could predict how well the siRNA will perform[9]. In this study, the results of MTT showed that the inhibition efficacy of siRNA4 was better than the others at concentrations of 10, 20, 40, and 80 nmol/L and close to siRNA2 at a concentration of 160 nmol/L. The robust inhibition of hTERT mRNA and protein expression was observed in siRNA4 and siRNA2, respectively. From the above results, we proved that siRNA2 and siRNA4 had better activity than the other three. As siRNA4 targeted the same sequence screened by the full length gene targeting technique, it is suggested that if a site is available for hybridization to an RNase H inducible oligonucleotide, then it is also available for hybridization and cleavage by the siRNA complex.
Many of the siRNAs reported to date are designed to target coding sequences, typically selected sequences located 100-200 bases away from the translation initiation sequence AUG, avoiding 5’ or 3’ untranslated regions[10]. Recently it has been reported that siRNA located at 3’ untranslated region successfully inhibited the expression of target gene[11]. In our study, siRNA2 screened by random selection was located in hTERT translated region and exhibited inhibitory activity on hTERT gene expression. However, siRNA4 screened by the full length gene targeting technique was located in 3’ untranslated region and exhibited similar activity compared with siRNA2. So it is more reasonable to select target sequences along with the full length mRNA of interested gene than to only select in the coding region.
In our previous study, a series of antisense oligonucleotides were designed based upon hTERT mRNA secondary structure. One of them, named cantide, has been demonstrated having robust inhibitory effects on tumor cell growth[12] and hTERT gene expression[5]. In this study, various siRNAs were introduced to HepG2 cells by liposome-mediated transfection and exhibited specific inhibitory effects on cell growth and hTERT gene expression. Compared to cantide, active siRNA exerted similar effects at much lower concentration. This result suggests that RNAi may be a promising gene-based therapy for cancer treatment[13].
Although, until recently, the siRNA produced by transcription or chemical synthesis in vitro only achieved a transient effect by using classic method such as liposome-mediated transfection[14], preparing siRNA by T7 transcription system in vitro is a rapid, simple and low-costing strategy suitable for screening the effective target sites[15]. In this research, five siRNAs targeting different sites of hTERT mRNA were designed and synthesized rapidly by T7 transcription system in vitro and two of them had high activity of gene-specific silencing effect. Our results suggest that siRNAs synthesized from a DNA template is a useful and effective way to specifically silence gene expression.
Science Editor Zhu LH Language Editor Elsevier HK
| 1. | Kyo S, Inoue M. Complex regulatory mechanisms of telomerase activity in normal and cancer cells: how can we apply them for cancer therapy? Oncogene. 2002;21:688-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3486] [Cited by in RCA: 3472] [Article Influence: 144.7] [Reference Citation Analysis (0)] |
| 3. | Leirdal M, Sioud M. Gene silencing in mammalian cells by preformed small RNA duplexes. Biochem Biophys Res Commun. 2002;295:744-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Yu JY, DeRuiter SL, Turner DL. RNA interference by expression of short-interfering RNAs and hairpin RNAs in mammalian cells. Proc Natl Acad Sci USA. 2002;99:6047-6052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 778] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 5. | Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6971] [Cited by in RCA: 7064] [Article Influence: 282.6] [Reference Citation Analysis (0)] |
| 6. | Elbashir SM, Harborth J, Weber K, Tuschl T. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods. 2002;26:199-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 836] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 7. | Hohjoh H. RNA interference (RNA(i)) induction with various types of synthetic oligonucleotide duplexes in cultured human cells. FEBS Lett. 2002;521:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Holen T, Amarzguioui M, Wiiger MT, Babaie E, Prydz H. Positional effects of short interfering RNAs targeting the human coagulation trigger Tissue Factor. Nucleic Acids Res. 2002;30:1757-1766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 505] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 9. | Shi Y. Mammalian RNAi for the masses. Trends Genet. 2003;19:9-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 204] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 10. | Sui G, Soohoo C, Affar el B, Gay F, Shi Y, Forrester WC, Shi Y. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc Natl Acad Sci USA. 2002;99:5515-5520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 859] [Cited by in RCA: 886] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 11. | McManus MT, Petersen CP, Haines BB, Chen J, Sharp PA. Gene silencing using micro-RNA designed hairpins. RNA. 2002;8:842-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 239] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 12. | Wang SQ, Lin L, Chen ZD, Lin RX, Chen SH, Guan W, Wang XH. Effect of antisense oliginucleotides targeting telomerase catalytic subunit on tumor cell proliferation in vitro. Chinese Sci Bulletin. 2002;47:993-997. |
| 13. | Wall NR, Shi Y. Small RNA: can RNA interference be exploited for therapy? Lancet. 2003;362:1401-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 135] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Tuschl T. Expanding small RNA interference. Nat Biotechnol. 2002;20:446-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 296] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 15. | Leirdal M, Sioud M. Gene silencing in mammalian cells by preformed small RNA duplexes. Biochem Biophys Res Commun. 2002;295:744-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |