Published online Apr 28, 2005. doi: 10.3748/wjg.v11.i16.2491
Revised: April 2, 2004
Accepted: May 29, 2004
Published online: April 28, 2005
AIM: To investigate the combination effect of hTERT antisense oligonucleotide “Cantide” and three chemotherapeutic drugs (cisplatin, 5-fluorouracil (5-FU) and adriamycin (ADM)) on inhibiting the proliferation of HepG2, BGC and A549 cell lines in vitro, and to investigate the efficacy of Cantide used in combination with cisplatin (DDP) in vivo.
METHODS: Cantide was transfected into these tumor cells by Lipofectin, and cell growth activity was calculated by microcytotoxicity assay. In vivo study, cells of HepG2 were implanted in Balb/c nude mice for 4 d. Then Cantide, DDP and Cantide+DDP were given intraperitoneally for 24 d respectively. The body weights of the tumor-bearing animals and their tumor mass were measured later to assess the effect of combination therapy in the nude mice. To evaluate the interaction of Cantide and these chemotherapeutic drugs, SAS software and Jin Zhengjun method were used.
RESULTS: Combination treatments with 0.1 μmol/L Cantide reduced the IC50 of DDP, 5-FU and ADM from 1.07, 4.15 and 0.29 μg/mL to 0.25, 1.52 and 0.12 μg/mL respectively. The inhibition ability of DDP, 5-FU and ADM respectively in combination with Cantide in these tumor cells was higher than that of these drugs alone (P<0.0001). And synergism (Q≥1.15) was observed at the lower concentration of DDP (≤1 μg/mL), 5-FU (≤10 μg/mL) and ADM (≤0.1 μg/mL) with combination of Cantide. In vivo, combination treatment with Cantide and DDP produced the greater growth inhibition of human liver carcinoma cells HepG2 in nude mice (0.65±0.19 g tumor) compared with that when only one of these drugs was used (Cantide group: 1.05±0.16 g tumor, P = 0.0009<0.001; DDP group: 1.13±0.09 g tumor, P = 0.0001<0.001).
CONCLUSION: These findings indicate that Cantide may enhance therapeutic effectiveness of chemotherapeutic drugs over a wide range of tumor cells in vitro, and the combination use of Cantide and DDP can produce much higher inhibition rates, as compared with when either of these drugs was used only in vivo.
-
Citation: Yang Y, Lv QJ, Du QY, Yang BH, Lin RX, Wang SQ. Combined effects of Cantide and chemotherapeutic drugs on inhibition of tumor cells’ growth
in vitro andin vivo . World J Gastroenterol 2005; 11(16): 2491-2496 - URL: https://www.wjgnet.com/1007-9327/full/v11/i16/2491.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i16.2491
In some areas of the world, cancer has become or shortly will become the leading disease-related cause of death in human beings[1]. In contrast, there are still many inadequate medical treatments of cancer. The main curative therapies for cancer surgery and radiation can be only successful in general if the cancer is found at an early stage and is localized. Currently conventional chemotherapy for treatment of the advanced tumors, although quite effective, has been associated with toxicities to normal tissue and organs, which is still a major dose limited factor. And chemoresistance is another major obstacle for successful treatment of cancer[2]. So it is difficult to remove these tumor cell contaminants with the use of the conventional chemotherapy only. It is clear that new therapeutic options are necessary.
Recent progresses in identification and characterization of new molecular targets for cancer and the limited effectiveness of conventional treatment strategies have attracted considerable attention on the development of new types of anticancer drugs. These new drugs would be highly specific for malignant cells with minimized side effects due to well-defined mechanisms of action. Antisense oligodeoxynucleotides (ASODNs) are a new drug they are short, synthetic stretches of DNA which can hybridize with specific mRNA strands that correspond to target genes. By binding to the mRNA, ASODN prevents the sequence of the target gene from being converted into a protein, thereby blocking the action of the gene[3]. So ASODNs have been extensively considered for the down regulation of oncogenes in cancer therapy[4]. And a more recent approach was the use of ASODNs in combination with conventional chemotherapy for potential anticancer therapy[5-7]. Combination therapy of ASODNs and cytotoxic drugs potentially has several advantages including lower doses of chemotherapeutic drugs, less side effects on normal cells and loss of chemoresistance. Hopefully the combination therapy will serve as a base for more effective elimination of tumor cells[8].
In our previous studies it was demonstrated that tumor cells treated with Cantide, an ASODN targeted to human telomerase reverse transcriptase (hTERT) mRNA, resulted in a relatively rapid decrease of tumor cells’ growth in vitro. Cytotoxic effect of Cantide was also compared with the sense, random and mismatched ODNs as control sequences. Only Cantide has potent inhibitory effect on tumor cells proliferation[9]. In vivo treatment of HepG2 tumor xenografts with Cantide significantly retarded the growth of the tumors. In this study, to explore the potential of Cantide used in combination with chemotherapeutic drugs on inhibition of human tumor cells’ growth, the cytotoxic interaction between Cantide and three chemotherapeutic drugs cisplatin (DDP), 5-fluorouracil (5-FU) and adriamycin (ADM) are analyzed in vitro and investigation of the efficacy of Cantide in combination with DDP in vivo is presented.
Human hepatocellular carcinoma cell (HepG2) was obtained from American Type Culture Collection. Human lung adenocarcinoma cells (A549) and human gastric cells (BGC823) were obtained from Chinese National Cancer Institute, Chinese Academy of Medical Science. HepG2 and BGC823 cells were cultured in DMEM (GIBCO BRL, Grand Island, NY, USA), supplemented with 10% FCS (GIBCO BRL), 100 U/mL penicillin and 100 U/mL streptomycin. A549 were cultured in RPMI-1640 (GIBCO BRL), supplemented with 10% FBS (GIBCO BRL), 100 U/mL penicillin and 100 U/mL streptomycin. Tumor cells were kept at 37 °C in a humidified atmosphere containing 50 mL/L CO2.
Cisplatin (DDP) was obtained from Qilu Pharmaceutical General Factory, China. 5-FU was obtained from Tianjin people’s Pharmaceutical Factory, China. ADM was obtained from Hisun Pharmaceutical Co. Ltd, China.
The antisense phosphorothioate oligodeoxynucleotides “Cantide” (5’-ACTCACTCAGGCCTCAGACT-3’) was synthesized on solid supports using Oligo Pilot II DNA (Amersham-Pharmacia, USA) and purified by HPLC Prep 4000 (Waters Delta, USA) with SOURCE 15Q (Amersham-Pharmacia, USA).
HepG2 cells were seeded at a density of 4×103 cells/well flat-bottomed plates (100 µL/well). After 24 h, culture medium was removed and the cells were washed with fresh FCS-free DMEM. In the above-mentioned medium, Cantide was delivered into these cells in the form of complex with Lipofectin (Invitrogen, USA) as described in the direction of Lipofectin. Using this method, three samples of concentrations (0.1, 0.2, 0.4 µmol/L) of Cantide were transfected into HepG2. After incubating for 6 h, 100 µL of cell culture medium with different chemotherapeutic drugs was replaced in each well. And five concentrations around the IC50 of each chemotherapeutic drugs (DDP: 0.25, 0.5, 1, 2, 4 µg/mL; 5-FU: 1.0, 2.5, 5, 10, 20 µg/mL; ADM: 0.025, 0.05, 0.1, 0.2, 0.4 µg/mL) were used. Each anticancer drugs’ different concentrations was used in combination with Cantide’s three concentrations (0.1, 0.2, 0.4 µmol/L) respectively, and each test group was tested thrice. At the same time, treatments with Cantide alone and either of the anticancer drugs were assayed, and treatments with cell culture medium without any drugs were used as control tests. After 72-h incubation, 20 µL of MTS (Promega, USA) was added in each well, followed by a 90-min incubation at 37 °C. Inhibition rate (IR) of tumor cells proliferation was assessed according to absorption at 490 nm using a Victor 1420 Multilable Counter (WALLAC, USA).
Human hepatocellular carcinoma cells (HepG2), human lung adenocarcinoma cell lines (A549) and human gastric tumor cells (BGC823) were tested and results obtained are presented in this paper. A549 and BGC823 cells were treated as what was done with HepG2 cells (as described above), but the ranges of concentrations of anticancer drugs were different according to different cell lines.
Female 4-5-wk-old Balb/c nude mice were purchased from Center for Animals for Experiment, Chinese Academy of Medical Science. HepG2 tumor cells cultured in vitro and 6×106 cells were injected into the neck of the nude mice. Four days later, the tumor could be sensed by touch.
The above-mentioned nude mice were divided into four groups (n = 7-8 mice/group). Cantide only group: Cantide was dissolved in saline and administered intraperitoneally (i.p.) 50 mg/kg daily for 24 consecutive days; DDP only group: DDP was dissolved in saline and administered intraperitoneally (i.p.) 1 mg/kg every other day for seven times totally; Combination treatment group: the nude mice were treated by Cantide plus DDP as described above; and negative control group (saline, i.p.). Tumor size was measured in two dimensions by calipers every 3 d, and the volume was calculated as length×width2×0.52. The nude mice were then killed and the solid tumors were peeled off on the 27th d after treatment. Then their tumors were weighed and IR calculated.
The software package for statistical analyses was SAS 6.12, and factorial design was used in the treatments in vitro, while t test was performed to assess potentially significant differences between individual groups of observations in vivo. In all tests, the significance of differences was accepted at P<0.05.
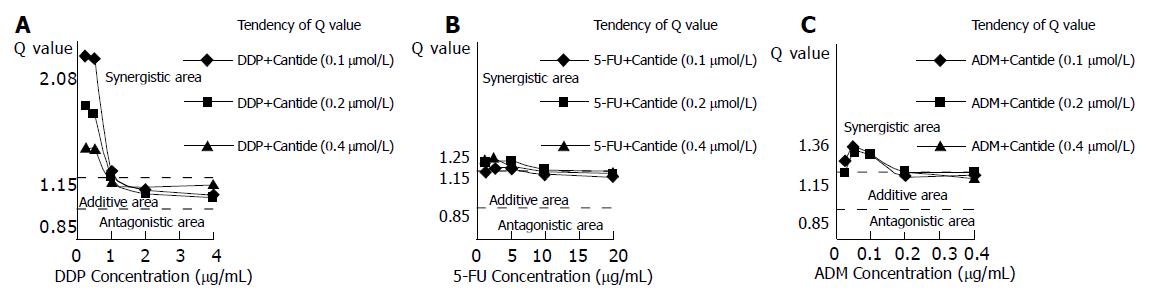
To analyze the interaction between Cantide and the three anticancer drugs, Zheng-Jun Jin method[10] was used. This method provides a “Q” value, according to which the interaction between two drugs can be classified as antagonistic effect (Q≤0.85), additive effect (0.85≤Q<1.15) or synergistic effect (Q≥1.15). And the formula is Q = Ea+b/(Ea+Eb-Ea×Eb), where Ea+b, Ea and Eb are average effect of combination treatment, effect of drug A only and effect of drug B only, respectively.
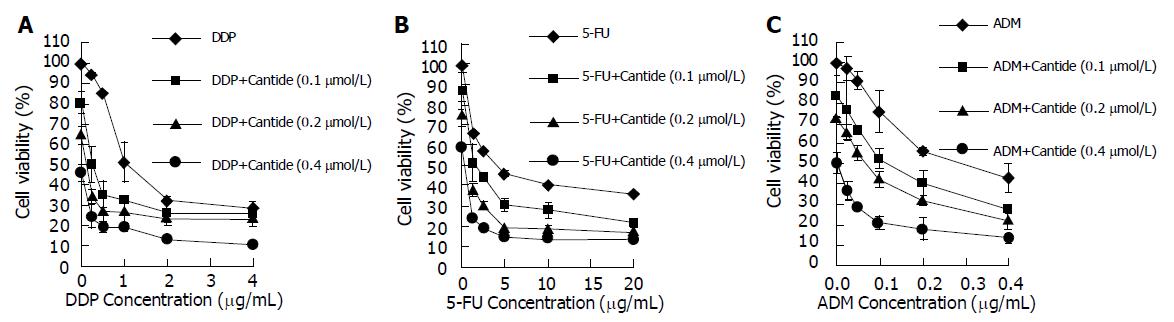
Cantide can decrease proliferation of HepG2 and increase HepG2 cells’ sensitivity to anticancer treatment. For each experiment, dose-response curves of each single chemotherapeutic drug and its combination with Cantide at different doses were performed. Figure 1A shows that the drug concentration causing 50% growth inhibition (IC50) of treatment of HepG2 cells with DDP only is 1.00 μg/mL, and IC50 of treatment of HepG2 cells with DDP in combination with Cantide (0.1 μmol/L) is 0.25 μg/mL. On the other hand, DDP can also increase the efficacy of Cantide. For example, 0.1 μmol/L Cantide used only (the dot which corresponds to 0 concentration of DDP on the dose-response curves) and when it was used in combination with 0.5 μg/mL DDP reduced the cell viability from 80% to 35%. Figure 1B indicates that combination treatment with 0.1 μmol/L Cantide reduced the IC50 of 5-FU from 4.15 to 1.52 μg/mL. And combination treatment with 0.1 μmol/L Cantide reduced the IC50 of ADM from 0.29 to 0.12 μg/mL (Figure 1C). The dose-response curves obtained from the combination experiments indicate that Cantide increased the cytotoxicity of DDP, 5-FU and ADM on HepG2 cells. And analysis with SAS software demonstrates statistically significant differences between any of the various single-drug treatments and combination treatment as indicated (P<0.0001).
To investigate the nature of the interaction between Cantide and the anticancer drugs on HepG2 cells, Zheng-Jun Jin method[10] was used to analyze the cytotoxicity data for antagonism, additivity or synergy. Q values in Figure 2 indicate that the synergistic effects appeared for the combinations of Cantide with lower concentrations of anticancer drugs. Furthermore, Figure 2A shows that the combination treatments of DDP plus Cantide obtained better synergistic effects than those of other anti-drugs’ combinations. The top Q value for combination treatment of Cantide and DDP was 2.08.
It was confirmed that the reduced cell growth rate in a variety of carcinoma cells upon treatment with single drug or the various combination treatments was due to the induction of cell death and cell viability calculated with microcytotoxicity assay (MTT) assays. As shown in Table 1, these values represent IC50 of chemotherapeutic drugs only and those of various combinations with Cantide on BGC and A549 cells. Values in first column with concentration treatments of Cantide are 0.0 (μmol/L) represent treatments with chemotherapeutic drugs only. And values in the next three columns represent IC50 of chemotherapeutic drugs in combination with Cantide. Similar results were obtained in cultures with BGC and A549 cells that combination treatments could decrease the IC50 of anticancer drugs.
| Tumor cell lines | Chemotherapeuticdrugs | Concentration of Cantide (mmol/L) | |||
| 0.0 | 0.1 | 0.2 | 0.4 | ||
| DDP | 0.45 | 0.36 | 0.27 | 0.15 | |
| BGC | 5-FU | 10.28 | 7.84 | 2.17 | 1.70 |
| ADM | 0.60 | 0.43 | 0.38 | 0.11 | |
| DDP | 5.43 | 3.21 | 2.04 | 0.96 | |
| A549 | 5-FU | 9.69 | 9.06 | 5.34 | 0.31 |
| ADM | 0.76 | 0.71 | 0.60 | 0.43 | |
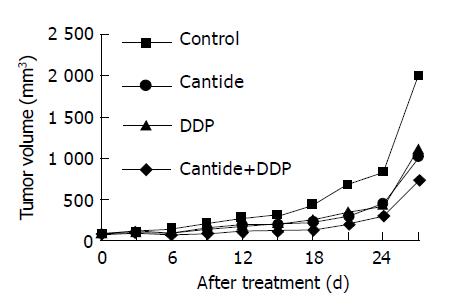
On the 4th d after injecting HepG2 cell into these nude mice, the tumor could be sensed by touching and the volumes of tumors were approximately equal. Then these mice were divided into four treatment groups: DDP only, Cantide only, DDP+Cantide and control group. Six days later, the differences between the average tumor volume of the control group and each of those of the three treatment groups were statistically significant respectively (all P<0.05). And the tumor volume of combination therapy group increased less than that of any other treatment groups. Twelve days later, the volume of tumor in the combination treatment group was significantly smaller than those in the single drug therapy groups (P<0.05). When the test came to the end, the tumor volume of all groups were as follows: control, 1995.21±342.77 mm2; DDP 1 mg/kg, 1107.07±222.66; Cantide 50 mg/kg, 1022.88±284.70 mm2; DDP 1 mg/kg+ Cantide 50 mg/kg, 729.86±128.04 mm2 (P<0.01) (Figure 3). After these nude mice were killed, the solid tumors were peeled off to measure their weights and calculate the IR. The IR of combination therapy was 70.0% and it was significantly greater than the IR of the single drug therapy groups (P<0.001), where DDP and Cantide were 47.9% and 51.6% respectively (Table 2). And compared with single drug groups, the combination therapy had no significant side effect on nude mice.
| Group | Tumor weight (g) | Inhibitory rate (%) |
| Control | 2.17±0.31 | 0.0 |
| Cantide | 1.05±0.16 | 51.6 |
| DDP | 1.13±0.09 | 47.9 |
| Cantide+DDP | 0.65±0.19 | 70.0 |
Telomerase is an RNA-dependent DNA polymerase that synthesizes telomeric DNA sequences and almost universally provides the molecular basis for unlimited proliferation potential. Since first discovered in Tetrahymena thermophila in 1985[11], telomerase activity was found to be absent in most normal human somatic cells but present in over 90% of cancerous cells and in vitro immortalized cells[12,13]. The holoenzyme consists of two essential components: one is a functional RNA component (hTR)[14,15], which serves as a template for telomeric DNA synthesis; the other is a catalytic protein (hTERT) with reverse transcriptase activity[16-19]. hTR is highly expressed in all tissues regardless of telomerase activity[20], with cancer cells generally having five-fold higher expression than normal cells. On the contrary, the expression (mRNA) of hTERT is estimated at less than 1-5 copies per cell and is closely associated with telomerase activity in cells. hTERT is generally repressed in normal cells and upregulated in immortal cells, suggesting that hTERT is the primary determinant for the enzyme activity[21,22]. Thus, inhibition of hTERT function is expected to be particularly effective on tumors. And there are some studies which prove that inhibition of telomerase could increase the sensitivity of DNA damaging drugs to tumor cells[23,24]. So, based on our previous studies[9], we investigated the ability of Cantide, an ASODN against hTERT mRNA, to sensitize carcinoma cells to hemotherapy and searched for antisense-drug combinations that could produce synergistic cytotoxicity.
In this study, the cell lines HepG2, A549 and BGC were treated with Cantide, DDP, 5-FU or ADM only, or with different combinations of Cantide and chemotherapeutic drugs. As the example of HepG2 cells, the results showed that the inhibition ability of DDP, 5-FU and ADM respectively when used in combination with Cantide on HepG2 cells was higher than any of those when only one of the drugs was used (P<0.0001). And synergism (Q≥1.15) was observed for lower concentration of DDP (≤1 μg/mL), 5-FU (≤10 μg/mL) and ADM (≤0.1 μg/mL) for combination treatment with Cantide. In order to explore the inhibitory effects of combination treatments in different tumor cells further, the cell lines A549 and BGC were tested with the same methods as that for HepG2 cells. The data also demonstrated that combination treatment for these tumor cell lines with Cantide plus anticancer drugs synergistically induced greater growth inhibition of cancer cells when compared with treatment in which either one of the drugs was used. In vivo test, the results of the tumor growth delay assays were used for Cantide (50 mg/kg) only, DDP (1 mg/kg) only and Cantide (50 mg/kg)+DDP (1 mg/kg) in nude mice with intraperitoneal injection (i.p). The IR of tumor growth of combination therapy was significantly greater than that of any therapy using only one drug (P<0.001).
These findings could encourage further research in antisense therapy where Cantide is used in combination with chemotherapeutic drugs, and our conclusions are also supported by recent work of others showing the relationship between telomerase and chemotherapeutic drugs.
Cisplatin (DDP) is frequently prescribed for the treatment of a wide variety of neoplasms. It causes DNA strand break especially at guanine residues. Several possibilities exist as to how cisplatin might interfere with telomerase function[25]. One possibility is that the telomeric repeat sequence (TTAGGG) n could be cross-linked as G-Pt-G, A-Pt-G or G-Pt-T-Pt-G. Alternatively, interactions of cisplatin with essential sulfhydryl groups in the protein part of the enzyme are also possible. Furthermore, there is evidence that cisplatin might disable transcription of the telomerase-RNA encoding gene region, as the expression of human telomerase RNA component, measured using the hTR-specific TRC3 primers, was significantly decreased[26]. Since cisplatin’s effect on telomerase activity is distinct from other cytotoxic drugs as described above, one might propose that inhibition of telomerase activity could, in part, contribute to cisplatin’s remarkable efficacy against tumors and thus inhibition of telomerase activity might have therapeutic potential.
ADM promotes apoptotic cell death in a variety of experimental tumor cell lines[27]. It is found that ADM-induced DNA damage appears to preferentially target chromosome ends resulting in substantial telomere-related cytogenetic abnormalities, indicating that the observed senescence is due to telomere dysfunction[28].
5-FU, an inhibitor of thymidylate synthase, was shown to be more cytotoxic when used in combination with ASODN specific for thymidylate synthase mRNA. And Mitsui et al[29], found that some cancer cells exposed to 5-FU showed a diminished telomerase activity preceded by a time-dependent decrease in the mRNA expression of hTERT. Thus, therapeutic strategies involving applications of ASODN in combination with conventional cytotoxics appear promising for potential cancer therapy[30].
Although a critical and careful evaluation of telomere inhibitors in cancer chemotherapy in vivo is certainly required, and further studies on the mechanism of combination therapy are needed, our present data indicate that Cantide, the inhibitor of telomerase maintenance, may act to make cancers chemosensitize to DDP, 5-FU and ADM. Thus, it can encourage the development and evaluation of this therapeutic combination of drug applications.
| 1. | Gibbs JB. Mechanism-based target identification and drug discovery in cancer research. Science. 2000;287:1969-1973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 315] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 2. | Benner E, Bishop MR, Agarwal N, Iversen P, Joshi SS. Combination of antisense oligonucleotide and low-dose chemotherapy in hematological malignancies. J Pharmacol Toxicol Methods. 1997;37:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Jansen B, Zangemeister-Wittke U. Antisense therapy for cancer--the time of truth. Lancet Oncol. 2002;3:672-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 118] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Akhtar S, Hughes MD, Khan A, Bibby M, Hussain M, Nawaz Q, Double J, Sayyed P. The delivery of antisense therapeutics. Adv Drug Deliv Rev. 2000;44:3-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 150] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Gleave ME, Zellweger T, Chi K, Miyake H, Kiyama S, July L, Leung S. Targeting anti-apoptotic genes upregulated by androgen withdrawal using antisense oligonucleotides to enhance androgen- and chemo-sensitivity in prostate cancer. Invest New Drugs. 2002;20:145-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | van de Donk NW, Kamphuis MM, van Dijk M, Borst HP, Bloem AC, Lokhorst HM. Chemosensitization of myeloma plasma cells by an antisense-mediated downregulation of Bcl-2 protein. Leukemia. 2003;17:211-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Miyake H, Hara I, Kamidono S, Gleave ME. Novel therapeutic strategy for advanced prostate cancer using antisense oligodeoxynucleotides targeting anti-apoptotic genes upregulated after androgen withdrawal to delay androgen-independent progression and enhance chemosensitivity. Int J Urol. 2001;8:337-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Klasa RJ, Bally MB, Ng R, Goldie JH, Gascoyne RD, Wong FM. Eradication of human non-Hodgkin's lymphoma in SCID mice by BCL-2 antisense oligonucleotides combined with low-dose cyclophosphamide. Clin Cancer Res. 2000;6:2492-2500. [PubMed] |
| 9. | Wang SQ, Lin L, Chen ZB, Lin RX, Chen SH, Guan W, Wang XH. Effect of antisense oligonucleotides targeting telomerase catalytic subunit on tumor cell proliferation in vitro. Chinese Sci Bulletin. 2002;47:993-997. |
| 10. | Jin ZJ. Addition in drug combination (author's transl). Zhongguo YaoLi XueBao. 1980;1:70-76. [PubMed] |
| 11. | Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2289] [Cited by in RCA: 2369] [Article Influence: 57.8] [Reference Citation Analysis (5)] |
| 12. | Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5156] [Cited by in RCA: 5262] [Article Influence: 164.4] [Reference Citation Analysis (0)] |
| 13. | Tatsumoto N, Hiyama E, Murakami Y, Imamura Y, Shay JW, Matsuura Y, Yokoyama T. High telomerase activity is an independent prognostic indicator of poor outcome in colorectal cancer. Clin Cancer Res. 2000;6:2696-2701. [PubMed] |
| 14. | Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J. The RNA component of human telomerase. Science. 1995;269:1236-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1606] [Cited by in RCA: 1612] [Article Influence: 52.0] [Reference Citation Analysis (5)] |
| 15. | Ren X, Gavory G, Li H, Ying L, Klenerman D, Balasubramanian S. Identification of a new RNA.RNA interaction site for human telomerase RNA (hTR): structural implications for hTR accumulation and a dyskeratosis congenita point mutation. Nucleic Acids Res. 2003;31:6509-6515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Lai CK, Mitchell JR, Collins K. RNA binding domain of telomerase reverse transcriptase. Mol Cell Biol. 2001;21:990-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 141] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Kilian A, Bowtell DD, Abud HE, Hime GR, Venter DJ, Keese PK, Duncan EL, Reddel RR, Jefferson RA. Isolation of a candidate human telomerase catalytic subunit gene, which reveals complex splicing patterns in different cell types. Hum Mol Genet. 1997;6:2011-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 427] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 18. | Lue NF, Lin YC, Mian IS. A conserved telomerase motif within the catalytic domain of telomerase reverse transcriptase is specifically required for repeat addition processivity. Mol Cell Biol. 2003;23:8440-8449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Horikawa I, Barrett JC. Transcriptional regulation of the telomerase hTERT gene as a target for cellular and viral oncogenic mechanisms. Carcinogenesis. 2003;24:1167-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 156] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Avilion AA, Piatyszek MA, Gupta J, Shay JW, Bacchetti S, Greider CW. Human telomerase RNA and telomerase activity in immortal cell lines and tumor tissues. Cancer Res. 1996;56:645-650. [PubMed] |
| 21. | Yi X, Tesmer VM, Savre-Train I, Shay JW, Wright WE. Both transcriptional and posttranscriptional mechanisms regulate human telomerase template RNA levels. Mol Cell Biol. 1999;19:3989-3997. [PubMed] |
| 22. | Kyo S, Takakura M, Taira T, Kanaya T, Itoh H, Yutsudo M, Ariga H, Inoue M. Sp1 cooperates with c-Myc to activate transcription of the human telomerase reverse transcriptase gene (hTERT). Nucleic Acids Res. 2000;28:669-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 385] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 23. | Kondo Y, Kondo S, Tanaka Y, Haqqi T, Barna BP, Cowell JK. Inhibition of telomerase increases the susceptibility of human malignant glioblastoma cells to cisplatin-induced apoptosis. Oncogene. 1998;16:2243-2248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 119] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | He D, Zhang H. Antisense oligodeoxynucleotide of telomerase gene enhances cisplatin-induced apoptosis in acute myeloid leukemia and chronic myeloid leukemia cells. Zhonghua NeiKe ZaZhi. 2001;40:654-656. [PubMed] |
| 25. | Cheng H, Wu Z, Zheng J, Lu G, Yan J, Liu M, Huang D, Lin J. Inhibition on telomerase activity and cytotoxic effects by cisplatin in cultured human choroidal melanoma cells. YanKe XueBao. 2003;19:54-59. [PubMed] |
| 26. | Burger AM, Double JA, Newell DR. Inhibition of telomerase activity by cisplatin in human testicular cancer cells. Eur J Cancer. 1997;33:638-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 106] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Ladeda V, Adam AP, Puricelli L, Bal de Kier Joffé E. Apoptotic cell death in mammary adenocarcinoma cells is prevented by soluble factors present in the target organ of metastasis. Breast Cancer Res Treat. 2001;69:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Elmore LW, Rehder CW, Di X, McChesney PA, Jackson-Cook CK, Gewirtz DA, Holt SE. Adriamycin-induced senescence in breast tumor cells involves functional p53 and telomere dysfunction. J Biol Chem. 2002;277:35509-35515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 194] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 29. | Mitsui A, Kuwabara Y, Iwase H, Mitani M, Shinoda N, Sato A, Toyama T, Sugiura M, Suzuki T, Kato J. Telomerase activity in esophageal squamous cell carcinoma: down-regulation by chemotherapeutic agent. J Surg Oncol. 2002;79:37-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Ciardiello F, Caputo R, Troiani T, Borriello G, Kandimalla ER, Agrawal S, Mendelsohn J, Bianco AR, Tortora G. Antisense oligonucleotides targeting the epidermal growth factor receptor inhibit proliferation, induce apoptosis, and cooperate with cytotoxic drugs in human cancer cell lines. Int J Cancer. 2001;93:172-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
Co-first-authors: Ying Yang and Sheng-Qi Wang
Co-correspondents: Ying Yang and Sheng-Qi Wang
Science Editor Guo SY Language Editor Elsevier HK