Published online Apr 28, 2005. doi: 10.3748/wjg.v11.i16.2426
Revised: July 13, 2004
Accepted: September 4, 2004
Published online: April 28, 2005
AIM: To evaluate the anti-tumor effects and possible involvement of anti-tumor immunity of electrochemotherapy (ECT) employing electroporation and bleomycin in human colon cancer xenografts in nude mice, and to establish the experimental basis for clinical application of ECT.
METHODS: Forty nude mice, inoculated subcutaneously human colon cancer cell line LoVo for 3 wk, were allocated randomly into four groups: B+E+ (ECT), B+E- (administration of bleomycin alone), B-E+ (administration of electric pulses alone), and B-E- (no treatment). Tumor volumes were measured daily. The animals were killed on the 7th d, the weights of xenografts were measured, and histologies of tumors were evaluated. Cytotoxicity of spleen natural killer (NK) and lymphokine-activated killer (LAK) cells was then assessed by lactic dehydrogenase release assay.
RESULTS: The mean tumor volume of group B+E+ was statistically different from the other three groups after the treatment (F = 36.80, P<0.01). There was one case of complete response, seven cases of partial response (PR) in group B+E+, one case of PR in group B+E- and group B-E+ respectively, and no response was observed in group B-E-. The difference of response between group B+E+ and the other three groups was statistically significant (χ2 = 25.67, P<0.01). Histologically, extensive necrosis of tumor cells with considerable vascular damage and inflammatory cells infiltration were observed in group B+E+. There was no statistical difference between the cytotoxicity of NK and LAK cells in the four treatment groups.
CONCLUSION: ECT significantly enhances the chemosensitivity and effects of chemotherapy in human colon cancer xenografts in nude mice, and could be a kind of novel treatment modality for human colon cancer. The generation of T-cell-dependent, tumor-specific immunity might be involved in the process of ECT.
- Citation: Zheng MH, Feng B, Li JW, Lu AG, Wang ML, Hu WG, Sun JY, Hu YY, Ma JJ, Yu BM. Effects and possible anti-tumor immunity of electrochemotherapy with bleomycin on human colon cancer xenografts in nude mice. World J Gastroenterol 2005; 11(16): 2426-2430
- URL: https://www.wjgnet.com/1007-9327/full/v11/i16/2426.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i16.2426
Colorectal cancer (CRC) is the second leading cause of malignancy not only in the Western world but also in China[1]. Despite advances in therapy, the 5-year survival rate is no more than 50-60%, mainly because of the frequency of hepatic involvement. And only 10-15% such hepatic metastasis can be curatively resected. Furthermore, there are a considerable number of patients who cannot undergo surgery even at an early stage of disease due to severe complications, such as chronic heart failure, chronic renal failure, and chronic obstructive pulmonary disease, etc. Conventional chemotherapy with 5-fluorouracil may be of some value in individual cases but can be limited by side effects when used systemically. Therefore, the introduction of effective chemotherapy against CRC is urgently needed, especially for patients who cannot undergo surgery and have recurrent disease.
Electrochemotherapy (ECT) could provide an innovative therapeutic approach for the treatment of CRC. The combination treatment, which involves the administration of a chemotherapeutic drug followed by the delivery of electric pulses to tumors was termed ECT[2]. The electric pulse electroporates the tumor cell membrane transiently and reversibly, allowing the chemotherapeutic agents greater access to its intracellular site of action and consistently provides improved responses relative to treatment with drugs alone. Although various anticancer drugs have been employed in ECT, the cytotoxic effect of bleomycin has reportedly been more significantly enhanced by electroporation than that of any other anticancer drug[3-5]. This experimental study was designed to evaluate the in vivo anti-tumor effects of ECT employing electroporation and bleomycin in human colon cancer xenografts in nude mice and cytotoxicity of spleen natural killer (NK) and lymphokine-activated killer (LAK) cells was further assessed to elucidate the possible anti-tumor immunity of ECT.
Electrical pulses were derived from a pulse generator (ECM 830; The BTX Division of Genetronics, USA). The output voltage from this generator is between 5 and 2500 V, and pulses can be applied at a set voltage for a pulse length varying between 10 µs and 10 s. The electrode used in the study is BTX Tweezer Model 520 (Genetronics, USA) with a diameter of 7 mm and a pulse length between 10 µs and 99 ms.
LoVo, a metastatic human colon adenocarcinoma cell line, was obtained from American type culture collection (ATCC) and was maintained in RPMI-1640 (Gibco) supplemented with 2 mmol/L L-glutamine and 100 mL/L FCS at 37 °C in a humidified atmosphere containing 50 mL/L CO2. For transplantation, the cells were more than 95% viable, as tested with the trypan blue exclusion dye method.
Male Balb/c (nu/nu) nude mice, aged 4-6-wk old and weighing 17-20 g, were purchased from animal center of Shanghai Institute of Cancer Research (Shanghai, China). Animals were housed and cared for according to the guidelines of specific pathogen free in Shanghai Second Medical University. LoVo cells resuspended in RPMI-1640 at a concentration of 1×107/mL and 100 µL of the cell suspension was inoculated subcutaneously into left front limb of the mice. Tumors were allowed to grow for 3 wk up to about 2-5 mm in longer diameter.
A total of 40 nude mice in which human colon cancer xenograft was successfully established were allocated randomly into four groups (10 mice/group): (1) Group B+E+: ECT. After induction of general anesthesia by an intraperitoneal injection of 0.25% embutal, 50 µL bleomycin (Harbin, China) was injected into the tumor at the concentration of 1000 μmol/L. Three minutes later, eight 100-µs pulses (field strength 900 V/cm) with a 10-ms interval were applied to the tumor; (2) Group B+E-: administration of bleomycin alone after anesthesia of the mice as described previously; (3) Group B-E+: administration of electric pulses alone with the same parameters described previously; (4) Group B-E- (no treatment): no treatment was applied to the mice except for the induction of anesthesia. Tumor size was measured by a caliper daily, and tumor volume was estimated according to the formula: V = 0.5×ab2/2, where a is the largest diameter and b is the smallest diameter. All animals of each group were killed on the 7th d, the weights of xenografts were measured. The volume of xenograft before and after the treatment were compared and response to the treatment was evaluated as follows[6]: complete response (CR): disappearance of the tumor; partial response (PR): reduction of the tumor volume by 50% or more; stable disease (Stab): reduction of the tumor volume by less than 50% or progression by less than 25%; progressive disease (Prog): increase in tumor volume by more than 25%. The efficacy of treatment was evaluated by comparing the mean post-therapy tumor volume of the four groups.
Splenocytes (effector cells) were isolated from all mice by mechanical dissociation and lysing of erythrocytes. Splenocytes were fractionated by density centrifugation at 2 000 r/min for 20 min with Ficoll Hypaque and interface lymphocytes were obtained. YAC-1 cells (ATCC), a mouse lymphoma line sensitive to the cytotoxic activity of NK cells, were used as targets. YAC-1 cells were maintained in RPMI 1640 supplemented with 10% FCS. NK cell activity was determined in triplicate by lactic dehydrogenase (LDH) release assay. YAC-1 (1×105) were mixed with lymphocytes at final effector/target (E:T) ratios between 20:1 in 96-well U-bottom plates. The plates were lightly centrifuged at 500 r/min for 4 min and incubated for 4 h at 37 °C in 50 mL/L CO2. The plates were then centrifuged at 500 r/min for 4 min, and 100-µL aliquots of supernatants were transferred from all wells to a fresh 96-well flat-bottom plate. One hundred microliters of LDH of substrate was added to each well and incubated for 20 min at room temperature. Fifty microliters of 1 mol/L HCl of stop solution was added, and the absorbance was recorded at 490 nm. The percentage of specific lysis was calculated using the formula: % cytotoxicity (NK) = (experimental LDH release-effector cell spontaneous LDH release-target cell spontaneous LDH release)/(target cell maximum LDH release-target cell spontaneous LDH release) ×100%.
IL2 was added into the remaining lymphocytes at a final concentration of 1000 U/mL, then the lymphocytes were incubated for 72 h at 37 °C in 50 mL/L CO2. LDH release assay described previously was used for measuring LAK cell activity. % cytotoxicity (LAK) = (experimental LDH release-effector cell spontaneous LDH release-target cell spontaneous LDH release)/(target cell maximum LDH release-target cell spontaneous LDH release) ×100%.
On the 7th d of the treatment, mice were killed. The tumors were excised and fixed in 10% formaldehyde for 24 h, then embedded in paraffin. Sections were prepared for hematoxylin-eosin staining and histologic changes were evaluated by microscopy (40×).
The data is presented as the mean±SD. ANOVA was used to compare tumor volume and weight among the four study groups and Kruskal-Wallis test was used to compare the response rate among groups. Statistical analyses were performed using SPSS 11.0 software. P<0.05 was considered statistically significant.
All mice tolerated the treatment well and no mice died of electroporation. Local swelling and medium necrosis were observed after the treatment in the mice of group B+E+, but not in those of group B-E+, group B+E- and group B-E-.
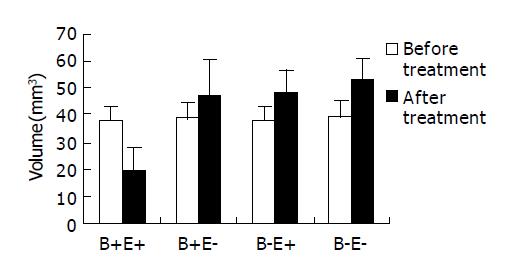
No significant difference was found among the four groups with respect to the mean tumor volume before treatment (Table 1). The tumor volume of group B+E+ on the 7th d of treatment was significantly reduced by 18.65±7.68 mm3 while that of other three groups were significantly increased by 8.09±12.53, 10.36±4.41 and 13.88±2.44 mm3 respectively (Figure 1). The tumor weight of group B+E+ was 14.48±6.65 mg, which was significantly less than that of other three groups. No significant difference was found among group B+E-, group B-E+, and group B-E- with respect to tumor volume and tumor weight (Table 1).
| Group | Mean tumor volume | Tumor volume variance (mm3) | Tumor weight aftertreatment (mg) | |
| Before (mm3) | After (mm3) | |||
| B+E+ | 37.92±5.39 | 19.28±8.831 | -18.65±7.681 | 14.48±6.651 |
| B+E- | 38.84±5.47 | 46.93±13.23 | 8.09±12.53 | 35.31±10.01 |
| B-E+ | 37.79±5.45 | 48.15±8.19 | 10.36±4.41 | 36.08±6.25 |
| B-E- | 39.32±6.30 | 53.20±7.22 | 13.88±2.44 | 39.99±5.41 |
| F value | F = 0.17 | F = 25.22 | F = 36.80 | F = 25.32 |
| P value | P = 0.917 | P<0.01 | P<0.01 | P<0.01 |
The response rates obtained after treatment are shown in Table 2. There was no statistically significant difference in the response rate among the first three groups (B-E-, B+E- and B+E+). In contrast, the response rate in group B+E+ differed significantly from that of each of the other group (P<0.01).
| Group | CR (%) | PR (%) | Stab (%) | Prog (%) |
| B+E+ | 1 (10) | 7 (70) | 2 (20) | 0 (0) |
| B+E- | 0 (0) | 1 (10) | 2 (20) | 7 (70) |
| B-E+ | 0 (0) | 1 (10) | 1 (10) | 8 (80) |
| B-E- | 0 (0) | 0 (0) | 0 (0) | 10 (100) |
Total splenocytes and activity of NK cells and LAK cells of each group are shown in Table 3. There was no statistically significant difference between group B+E+ and other three groups (B-E-, B+E- and B-E+) in total splenocytes and activity of NK cells and LAK cells.
| Group | Total splenocytes (106) | Activity of NK cells | Activity of LAK cells |
| B+E+ | 110.8±10.62 | 18.49±2.73 | 23.51±2.41 |
| B+E- | 114.3±10.68 | 18.89±2.57 | 25.21±3.09 |
| B-E+ | 116.4±10.32 | 18.68±2.86 | 25.32±2.90 |
| B-E- | 117.4±12.89 | 19.79±2.64 | 24.84±2.53 |
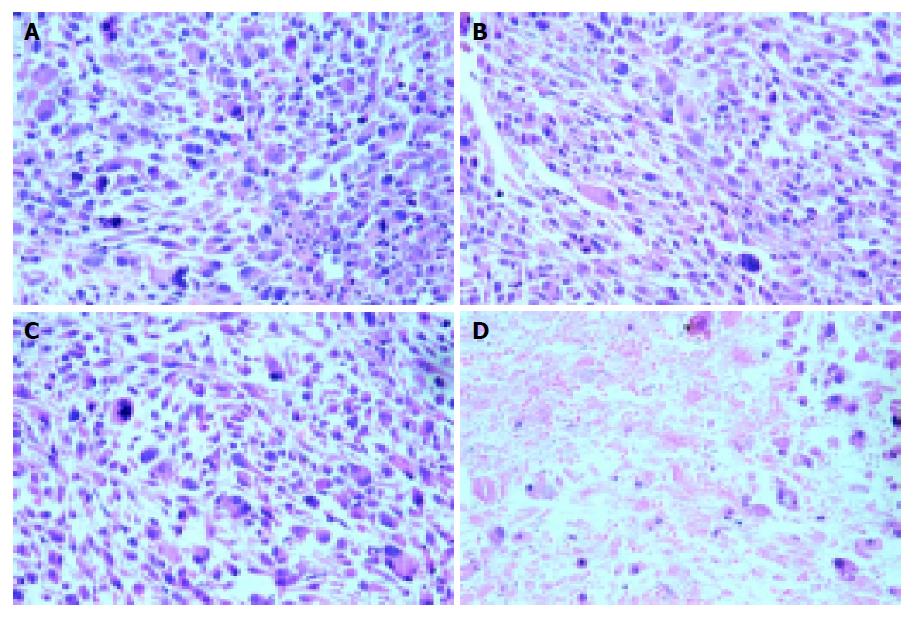
Undamaged tumor cells and a few accompanying infiltrating cells were observed not only in the tumors of group B-E- but also in that of group B+E- and group B+E+. In contrast, there were massive necrotic area with a moderately intense mononuclear infiltration in the tumors of group B+E+ (Figure 2). The one CR was also confirmed histologically.
ECT is a new cancer therapy that utilizes electric pulses to augment the effectiveness of chemotherapeutic agents. Destabilization of the lipid bilayer of the cell membrane by the electric pulses transiently increases permeability. This provides intracellular access for any exogenous molecules including bleomycin which must be internalized by a cell to be effective. Thus, the combination becomes a potent anti-tumor treatment. The effectiveness of ECT on various types of cancer has been demonstrated not only in vitro but also in animal models, and several clinical trials of ECT have already been initiated[7-10]; however, the possible involvements of anti-tumor mechanisms, especially that of anti-tumor immunity, remain still unclear.
In current study, the mean tumor volume and tumor weight of group B+E+ on the 7th d of treatment was significantly less than those of other three groups (P<0.01), and bleomycin-mediated ECT produced a tumor response rate of 80% (10% CR and 70% PR) which was significantly higher than those of other three groups (P<0.01). These results concur with other experimental studies and demonstrate that the bleomycin-mediated ECT is effective and sensitive to human colon cancer xenografts in nude mice. Furthermore, no mice died of electroporation in this study, which suggests that ECT using the voltage of 900 V/cm is safe and feasible.
Increased cytotoxicity of bleomycin by electroporation is the predominant mechanism involved in anti-tumor effectiveness of ECT. This notion is supported by the data demonstrating that exposure of tumor cells to electric pulses profoundly increased not only intracellular amounts of bleomycin but also intratumoral amounts of bleomycin several 100-fold[4,7,11]. Once inside the cell, bleomycin possesses a very potent intrinsic cytotoxicity, inducing single- and double-strand DNA breaks and cell cycle blockage[12]. Anti-vascular effect was also proposed as an additional mechanism involved in anti-tumor effectiveness of ECT[13]. Damage to endothelial cells caused by ECT may lead to obstruction of blood flow and ischemic death of tumor cells lining the obstructed blood vessel. It is supported by the observations that bleomycin-mediated ECT resulted in complete shut down of tumor blood flow observed 12 h after the treatment, and correlated with anti-tumor effectiveness and the extent of tumor necrosis[14]. Recently, ECT has been found to increase immune response to tumors[15-17] and the infiltration of inflammatory cells into tumor treated with bleomycin-mediated ECT was also reported[18]. However, its exact nature has yet to be defined.
NK cells recognize tumor cells without the need for immunization or pre-activation compared with T cells, which first require education by antigen-presenting cells[19,20]. Once activated, NK cells show increased cytolytic, secretory and proliferative functions. Activation of NK cells with high-dose IL-2 has been widely used and has been shown to mediate anti-tumor activity in experimental as well as clinical settings[21,22]. In current study, no statistically significant difference was found between group B+E+ and other three groups (B-E-, B+E- and B-E+) in total splenocytes and activity of NK cells and LAK cells, which demonstrated that non-specific immunity may not play an important role in the anti-tumor immunity of bleomycin-mediated ECT. Furthermore, it was reported that the induction of cytotoxic T lymphocytes against Colon 26 cells was observed in the spleens of mice with Colon 26 after treatment with ECT and bleomycin[16], and that the Balb/c mice inoculated subcutaneously with Colon 26 cells rejected inoculations of rechallenged Colon 26 cells after treatment of ECT and bleomycin, but not other kind of cancer cells[17]. Therefore, the generation of T-cell-dependent, tumor-specific protective immunity might be involved in the process of tumor nodule regression following ECT.
In conclusion, we have shown that in vivo ECT with bleomycin is effective in reducing or even eliminating subcutaneous human colon cancer xenografts in nude mice. Additional investigations are also required to define parameters such as the optimum duration of treatment, dose effects, and the volume of tumor that can be treated. Research is currently in progress in this area. If results are encouraging, a clinical trial could be initiated to determine whether ECT is a valid alternative to systemic chemotherapy for patients with nonresectable metastases of malignancies, especially for those with hepatic metastasis of CRC.
| 1. | Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002;52:23-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2235] [Cited by in RCA: 2117] [Article Influence: 88.2] [Reference Citation Analysis (0)] |
| 2. | Mir LM, Orlowski S, Belehradek J, Paoletti C. Electrochemotherapy potentiation of antitumour effect of bleomycin by local electric pulses. Eur J Cancer. 1991;27:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 424] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 3. | Gehl J, Skovsgaard T, Mir LM. Enhancement of cytotoxicity by electropermeabilization: an improved method for screening drugs. Anticancer Drugs. 1998;9:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 162] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Kuriyama S, Matsumoto M, Mitoro A, Tsujinoue H, Nakatani T, Fukui H, Tsujii T. Electrochemotherapy for colorectal cancer with commonly used chemotherapeutic agents in a mouse model. Dig Dis Sci. 2000;45:1568-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Rodríguez-Cuevas S, Barroso-Bravo S, Almanza-Estrada J, Cristóbal-Martínez L, González-Rodríguez E. Electrochemotherapy in primary and metastatic skin tumors: phase II trial using intralesional bleomycin. Arch Med Res. 2001;32:273-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Chazal M, Benchimol D, Baqué P, Pierrefite V, Milano G, Bourgeon A. Treatment of hepatic metastases of colorectal cancer by electrochemotherapy: an experimental study in the rat. Surgery. 1998;124:536-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Kuriyama S, Tsujinoue H, Toyokawa Y, Mitoro A, Nakatani T, Yoshiji H, Tsujimoto T, Fukui H. A potential approach for electrochemotherapy against colorectal carcinoma using a clinically available alternating current system with bipolar snare in a mouse model. Scand J Gastroenterol. 2001;36:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Mitsui K, Kato K, Nakamura K, Yamada Y, Honda N, Fukatsu H, Yoshikawa K. Effective treatment of bladder tumor-bearing mice by direct delivery of bleomycin using electrochemotherapy. Drug Deliv. 2002;9:249-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Kitamura A. Bleomycin-mediated electrochemotherapy in mouse NR-S1 carcinoma. Cancer Chemother Pharmacol. 2003;51:359-362. [PubMed] |
| 10. | Shimizu T, Nikaido T, Gomyo H, Yoshimura Y, Horiuchi A, Isobe K, Ebara S, Takaoka K. Electrochemotherapy for digital chondrosarcoma. J Orthop Sci. 2003;8:248-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Jaroszeski MJ, Dang V, Pottinger C, Hickey J, Gilbert R, Heller R. Toxicity of anticancer agents mediated by electroporation in vitro. Anticancer Drugs. 2000;11:201-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 120] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Mir LM, Tounekti O, Orlowski S. Bleomycin: revival of an old drug. Gen Pharmacol. 1996;27:745-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 146] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Sersa G, Cemazar M, Miklavcic D, Chaplin DJ. Tumor blood flow modifying effect of electrochemotherapy with bleomycin. Anticancer Res. 1999;19:4017-4022. [PubMed] |
| 14. | Sersa G, Krzic M, Sentjurc M, Ivanusa T, Beravs K, Kotnik V, Coer A, Swartz HM, Cemazar M. Reduced blood flow and oxygenation in SA-1 tumours after electrochemotherapy with cisplatin. Br J Cancer. 2002;87:1047-1054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Mir LM, Roth C, Orlowski S, Quintin-Colonna F, Fradelizi D, Belehradek J, Kourilsky P. Systemic antitumor effects of electrochemotherapy combined with histoincompatible cells secreting interleukin-2. J Immunother Emphasis Tumor Immunol. 1995;17:30-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Kuriyama S, Mitoro A, Tsujinoue H, Toyokawa Y, Nakatani T, Yoshiji H, Tsujimoto T, Okuda H, Nagao S, Fukui H. Electrochemotherapy can eradicate established colorectal carcinoma and leaves a systemic protective memory in mice. Int J Oncol. 2000;16:979-985. [PubMed] |
| 17. | Miyazaki S, Gunji Y, Matsubara H, Shimada H, Uesato M, Suzuki T, Kouzu T, Ochiai T. Possible involvement of antitumor immunity in the eradication of colon 26 induced by low-voltage electrochemotherapy with bleomycin. Surg Today. 2003;33:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Jaroszeski MJ, Coppola D, Pottinger C, Benson K, Gilbert RA, Heller R. Treatment of hepatocellular carcinoma in a rat model using electrochemotherapy. Eur J Cancer. 2001;37:422-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2:850-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 560] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 20. | Colucci F, Caligiuri MA, Di Santo JP. What does it take to make a natural killer? Nat Rev Immunol. 2003;3:413-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 354] [Article Influence: 15.4] [Reference Citation Analysis (1)] |
| 21. | Sacchi M, Vitolo D, Sedlmayr P, Rabinowich H, Johnson JT, Herberman RB, Whiteside TL. Induction of tumor regression in experimental model of human head and neck cancer by human A-LAK cells and IL-2. Int J Cancer. 1991;47:784-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Ueda Y, Yamagishi H, Tanioka Y, Fujiwara H, Fuji N, Itoh T, Fujiki H, Yoshimura T, Oka T. Clinical application of adoptive immunotherapy and IL-2 for the treatment of advanced digestive tract cancer. Hepatogastroenterology. 1999;46 Suppl 1:1274-1279. [PubMed] |
Science Editor Guo SY Language Editor Elsevier HK