INTRODUCTION
Liver fibrosis, which is characterized by the excess deposition of extracellular matrix components such as collagen I, III, and IV[1,2], is usually the ultimate pathological outcome for the majority of chronic liver injuries e.g., viral and alcoholic hepatitis. Central to the process of liver fibrosis is the activation of the hepatic stellate cells (HSC), which proliferates and stimulates the production and deposition of collagen. The activated HSC also express tissue inhibitors of metalloproteinase-1 (TIMP-1), which inhibit the degradation of interstitial collagens by interstitial collagenase such as matrix metalloproteinase-1 (MMP-1)[3-5].
Advanced fibrosis and cirrhosis were previously considered to be irreversible conditions. However, there is accumulating evidence that this fibrosis is reversible. Recovery, with remodeling of excess collagens, has been demonstrated in experimentally-induced fibrosis in animals[6] and in liver fibrosis in humans[7]. In situations of spontaneous recovery from liver fibrosis, there is a diminution of TIMP expression and an increase in collagenase activity with consequent degradation of the collagen matrix[8]. Furthermore, an additional finding in this and other studies, was that HSC apoptosis was a key process in the resolution of liver fibrosis[8-11]. However, while the loss of activated HSC can explain a decrease in TIMP expression, it is not in itself sufficient to explain the constitutive MMP-1 expression and continuous increase in collagenase activity, which remodels the existing excess collagens, because the number of activated HSC is dramatically reduced by one-tenth by apoptosis 7 d after peak fibrosis[8]. This cumulative evidence (the loss of HSC, but constitutive MMP-1 expression and an increase in the collagenase activity in the entire liver), suggests that hepatic parenchymal cells, the hepatocytes, might also be contributing to the resolution of liver fibrosis. We previously reported that fibril type I collagen stimulated MMP-1 expression in hepatocellular carcinoma tissue culture cell lines, such as HepG2 and HLE[12]. We have also confirmed that primary hepatocytes express both MMP-1 and TIMP-1 as well as HLE cells. In this study, we used fibril collagen, as a collagen degradation product, while fixed collagen was used to represent immobilized collagen as part of the extracellular matrix in liver fibrosis. We report here on our investigations into the effects of fibril- or fixed-collagen on MMP-1 and TIMP-1 production in HLE cells in order to establish what role liver parenchymal cells may play in the regulation of liver fibrosis.
MATERIALS AND METHODS
Cell culture
HLE cells, a human hepatocellular carcinoma derived cell line, were obtained from the Japanese Cancer Research Resource Bank. The cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Nissui Pharmaceutical Ltd., Tokyo, Japan) supplemented with 20% fetal calf serum (FCS), 200 units/mL penicillin, and 200 mg/mL streptomycin.
Experiment 1
The first experiment was designed to test the effect of fibril collagen on type I collagenase activity, TIMP-1 production, and MMP-1 production in HLE cells, cultured on a dish with or without collagen coating. To make fibril collagen, type I or type IV collagen (Nitta Gelatin Co. Ltd., Osaka, Japan) were treated with HCl (pH 3.0) at room temperature overnight, and neutralized with phosphate buffered saline (PBS). The procedure for coating the tissue culture dishes with collagen was as follows: 1 mL of fibril collagen solution (10 μg/mL) was placed in a 35 mm dish, and then exposed to ultraviolet (UV) light for 2 h. The coated dish was washed twice with FCS-free DMEM.
HLE cells were plated at a concentration of 5×106 and cultured with DMEM, supplemented with 20% FCS, until they reached confluency. They were then washed twice with FCS-free DMEM and cultured with 2 mL of FCS-free DMEM for 12 h. After washing the dishes with PBS, fibril type I or type IV collagen were added to FCS-free DMEM (final concentration: 0, 37.5, 125, 375, or 1250 μg/mL) and the cells were cultured for a further 24 h. The resulting conditioned (supernatant) medium was concentrated 15-fold for the assay of collagenase activity, and TIMP-1 and MMP-1 production.
Experiment 2
The second experiment was designed to evaluate the effects of coated, fixed collagen on MMP-1 and TIMP-1 production in HLE cells. The cells were prepared as described in experiment 1, and then cultured on dishes coated with 1 mL of several concentration of fibril collagen solution (0, 10, 30, or 100 μg/mL). The concentration of fibril type I or type IV collagen added in FCS-free DMEM was fixed at 37.5 μg/mL.
Measurement of type I collagenase activity
Type I collagenase activity was assayed using fluorescein isothiocyanate (FITC)-labeled type I collagen (0.1%/0.01 mol/L acetic acid) (Cosmo-Bio Co. Ltd, Tokyo, Japan) according to previously described methods[13]. Briefly, after trypsin activation, 50 μL of the concentrated conditioned medium was incubated with 100 μL of FITC-labeled type I collagen for 3 h at 35 °C. The reaction was terminated by the addition of 80 mmol/L O-phenanthrolene. Collagenase activity was estimated by measuring the fluorescent intensity of the supernatant at 495 nm (excitation)/520 nm (emission). A unit of collagenase activity was defined as the amount of enzyme that degraded 1 μg of collagen/min.
Measurement of TIMP-1 and MMP-1
Monoclonal anti-human TIMP-1 antibody, monoclonal anti-human MMP-1, and peroxidase-conjugated Fab’ were kindly donated by Fuji Chemical Industries Ltd (Toyama, Japan). TIMP-1 and MMP-1 were measured by a one-step sandwich enzyme immunoassay as previously described[14-16]. Briefly, a sample was mixed with 10 mmol/L sodium phosphate buffer containing 50 ng/mL peroxidase-conjugated Fab’, 1% bovine serum albumin, 0.1% Tween 20, 0.1 mol/L NaCl, and 0.005% thimerosal, and then 100 μL of the solution was transferred to a microplate well, coated with the monoclonal antibody. The preparation was allowed to stand for 30 min at room temperature and then washed thrice with PBS. Peroxidase activity was assayed after 20 min incubation with a reaction mixture containing a 0.15 mol/L citric acid phosphate buffer, 0.5 mg/mL O-phenylenediamine and 0.02% H2O2. Absorbance was measured at 492 nm in a microplate reader.
Statistical analysis
All results were presented as the mean±SE. Data were analyzed by one-way ANOVA, and differences between groups were assessed by Scheffé’s test. A level of P<0.05 was accepted as statistically significant.
RESULTS
Effects of fibril collagen on type I collagenase activity, MMP-1 production, and TIMP-1 production
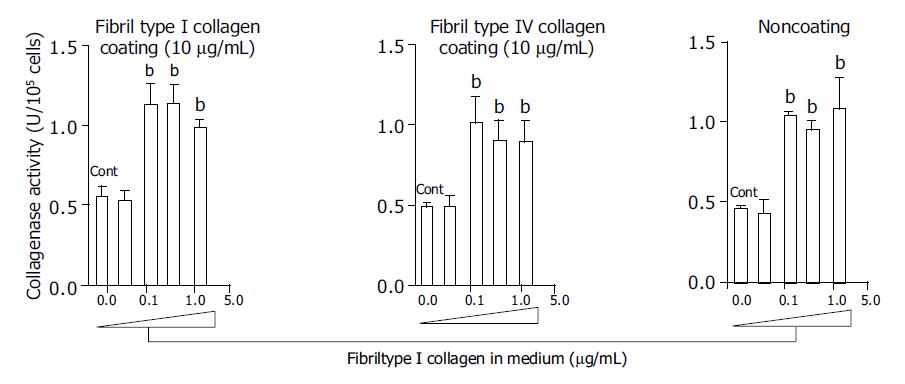
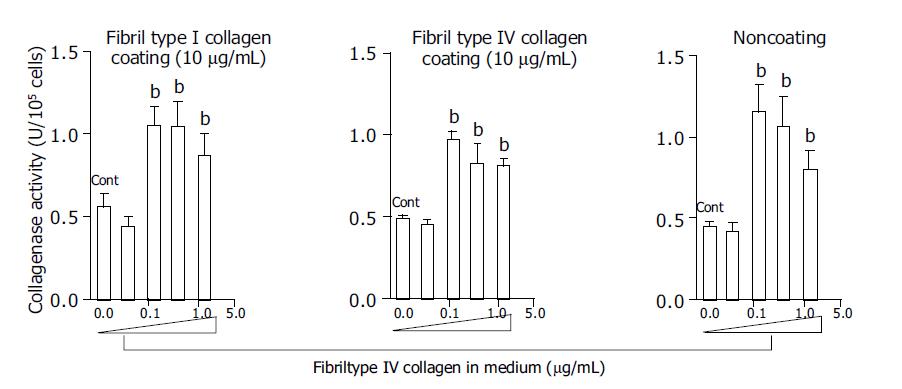
At a concentration of 125 μg/mL, fibril type I collagen significantly increased type I collagenase activity about two-fold compared with a concentration of 37.5 μg/mL or no fibril collagen (Figure 1). However, raising the concentration of fibril type I collagen to 375 or 1250 μg/mL did not further enhance the increased type I collagenase activity. The effects of the fibril collagen type I were not affected by the coating used, as results were similar for cells cultured with or without type I or type IV collagen coating (10 μg/mL) (Figure 1). Fibril type IV collagen had a similar effect on type I collagenase activity, and it was not affected by the coating conditions (Figure 2). There was no significant difference in the effects on collagenase activity between cells cultured in medium containing fibril type I collagen and those cultured in the presence of type IV collagen.
Figure 1 Effects of fibril type I collagen in the culture medium on the collagenase activity for type I collagen.
Culture dishes were coated with or without type I collagen (10 μg/mL) and type IV collagen (10 μg/mL). For the control, cells were cultured without fibril collagen. bP<0.01 vs controls (Cont).
Figure 2 Effects of fibril type IV collagen in the culture medium on collagenase activity for type I collagen.
Culture dishes were coated with or without type I collagen (10 μg/mL) and type IV collagen (10 μg/mL). For the control, cells were cultured without fibril collagen. bP<0.01 vs controls (Cont).
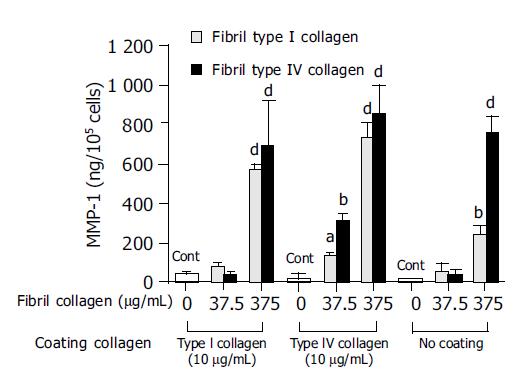
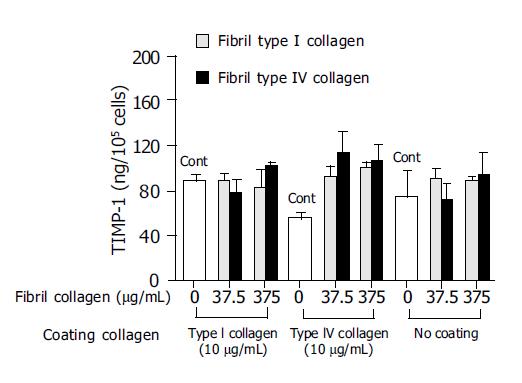
In order to investigate the mechanism of this enhanced type I collagenase activity by fibril type I or type IV collagen, the effects of the fibril collagens on MMP-1 production and TIMP-1 production were then evaluated. Both fibril type I and IV collagen significantly increased MMP-1 production, and at a concentration of 375 μg/mL both types showed more than 10-fold higher levels of MMP-1 than the control (Figure 3). The enhanced MMP-1 production by fibril collagens was unaffected by the presence or absence of coating with type I or type IV collagen (Figure 3). By contrast, TIMP-1 production was not changed by the addition of fibril type I or IV collagen, and neither was it affected by the coating conditions (Figure 4).
Figure 3 Effects of fibril type I (dotted bar) or type IV (filled bar) collagen on matrix metalloproteinase-1 (MMP-1) production.
For the control, cells were cultured without fibril collagen. aP<0.05; bP<0.01; dP<0.001 vs controls (Cont).
Figure 4 Effects of fibril type I (dotted bar) or type IV (filled bar) collagen on tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) production.
For the control(Cont), cells were cultured without fibril collagen.
Effects of the fixed type I collagen on MMP-1 and TIMP-1 production
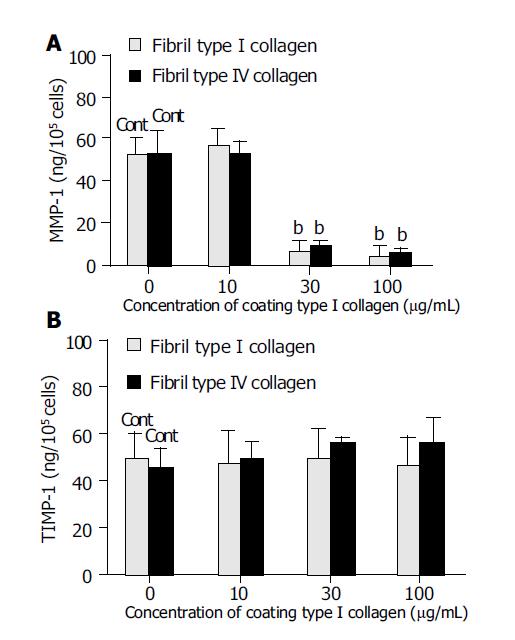
The effects of the amount of type I collagen coating (fixed collagen) on MMP-1 and TIMP-1 production were also evaluated. Coating with 30 or 100 μg/mL type I collagen significantly suppressed MMP-1 production by almost one-tenth compared with coating with 10 μg/mL type I collagen or no coating (Figure 5A). By contrast, TIMP-1 production was not affected by either the absence of a collagen coat or by increasing the concentration of the coating collagen (Figure 5B).
Figure 5 Effect of the coating concentrations of type I collagen on matrix metalloproteinase-1 (MMP-1) (A) and tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) (B) production.
For the control, cells were cultured on non-coating dishes. bP<0.01 vs controls (Cont).
DISCUSSION
We have demonstrated that fibril type I or IV collagen induced type I collagenase activity, and also increased MMP-1 production. However, neither type had an effect on TIMP-1 production. Type I collagenase activity is regulated by a balance between MMP-1 and TIMP-1, and our data indicated that the enhanced type I collagenase activity was attributable to increased MMP-1 production but not to decreased TIMP-1 production. The fact that the fibril collagens stimulated a 10-fold increase in MMP-1 but only a two-fold increase in the collagenase activity raises questions. One possible explanation is that we measured the whole MMP-1 protein, including pro-MMP1 that does not have any proteinase activity. Another explanation for this apparent discrepancy is that the difference may have been due to the presence of other members of the MMP or TIMP family, such as MMP-2, which is closely related to the process of tumor cell invasion[17]. However, although HLE cells are a hepatocellular carcinoma-derived cell line, in this study the collagenase activity was measured using type I collagen as a substrate, which is a target for MMP-1 not MMP-2. Consequently, the increased collagenase activity was assumed to reflect the up-regulation of MMP-1 activity. In normal cells, such as keratinocytes, MMP-1 expression is highly elevated after stimulation by type I collagen[18,19]. In this study, we used HLE cells as they are hepatocyte-derived cell lines, and have demonstrated increased MMP-1 production in response to fibril collagen[12]. Although we still need to establish that fibril collagen stimulates collagenase activity and MMP-1 production without affecting TIMP-1 production in primary hepatocytes, we have confirmed that rat primary hepatocytes expressed both MMP-1 and TIMP-1, and preliminarily found that fibril collagen stimulated MMP-1 mRNA expression (unpublished data). Therefore, the evidence from the results reported here, together with preliminary evidence in the rat primary hepatocytes, indicate that the up-regulation of MMP-1 by the fibril collagen is a common feature.
By contrast, fixed (coated) type I collagen down-regulated MMP-1, and did not affect TIMP-1 production. One could assume from this observed decrease in the production of MMP-1 and unchanged TIMP-1 production, that the collagenase activity would be down-regulated. Because collagen encountered in vivo, particularly in liver fibrosis, will commonly be immobilized as part of the extracellular matrix, we coated culture dishes with different, increasing concentrations of type I collagen. The precise mechanism behind the opposite effects seen between fibril- and fixed-collagen is unclear. However, it has been suggested that the conformation of fibril collagens interacting with HLE cells might be different from that of the immobilized fixed collagens. Integrins are important trans-cell membrane adhesion glycoproteins, with α and β subunits. They exist in an active and inactive form. The amino acid sequence arginine-glycine-aspartic acid (RGD) is a potent recognition sequence for integrin binding, including the binding of fibronectin to the α5β1 integrin receptor. The α1β1 and α2β1 integrins, both of which are expressed in HLE cells[20], are major cellular receptors for collagens and interact with the triple-helical region of most of the fibril collagen[21,22]. Binding of collagen to integrins is an essential, fundamental step in fibrogenesis and wound healing. We have reported previously that soluble RGD peptide reduced the accumulation of collagen in HSC by stimulating collagenase and MMP-1 activity[23], whereas, conversely, the fibronectin isoform, or EIII A segment, has been shown to increase collagen production[24]. Discoidin domain receptor 2 (DDR2) is another receptor for fibril collagen, and activation of DDR2 has been shown to induce the expression of MMP-1[25]. We have reported previously that HLE cells express the DDR2 receptor, and that fibril collagen stimulated MMP-1 production in these cells, whereas fixed collagen did not[15]. Taken together, all this evidence suggests that fibril collagen might induce MMP-1 production in HLE cells via its activation of DDR2.
Using HLE cells, we tried to evaluate the role of liver parenchymal cells in liver fibrosis and its resolution, although there is limitation of use of HLE cells even which express both MMP-1 and TIMP-1. During the process of liver fibrosis, HSC become active and produce immobilized collagens[1]. As shown in Figure 5, immobilized fixed collagen suppressed MMP-1 production but did not affect TIMP-1 production, which would lead to an overall accumulation of collagen in the liver. During the resolution of liver fibrosis, there is a diminution of TIMP expression and an increase in collagenase activity with consequence matrix degradation[11] including the formation of fibril collagen. These fibril collagens act on the hepatocytes to induce MMP-1 production, without affecting TIMP-1 production, resulting in induction of collagenase activity. This link could establish an autocrine-loop for the resolution of fibrosis. Since we know that HSC become apoptotic, and decrease in number by one-tenth within a week of the peak of fibrosis[11], the up-regulation of collagenase activity by hepatocytes could maintain the remodeling and resolution of fibrosis, even after the loss of HSC. Our data implicate hepatocytes as also playing an important role in both liver fibrosis and its resolution alongside HSC. Novel insights into the entire mechanism involved in the regulation of hepatic fibrosis may be obtained by studying the interactions of, or co-operation between, the various types of cells involved, such as HSC and hepatocytes. Furthermore, based on an understanding of the co-operation between various cell functions, such insights might lead to the development of improved therapeutic strategies for the various pathologies which are associated with the process of fibrosis.