Published online Mar 28, 2005. doi: 10.3748/wjg.v11.i12.1876
Revised: August 10, 2004
Accepted: October 18, 2004
Published online: March 28, 2005
AIM: To value whether omeprazole could induce the healing of DIS and regression of symptoms in patients with DGER.
METHODS: We enrolled 15 symptomatic patients with a pathological esophageal 24-h pH-metry and bilimetry. Patients underwent endoscopy and biopsies were taken from the distal esophagus. Specimens were analyzed at histology and transmission electron microscopy (TEM). Patients were treated with omeprazole 40 mg/d for 3 mo and then endoscopy with biopsies was repeated. Patients with persistent heartburn and/or with an incomplete recovery of DIS were treated for 3 more months and endoscopy with biopsies was performed.
RESULTS: Nine patients had a non-erosive reflux disease at endoscopy (NERD) while 6 had erosive esophagitis (ERD). At histology, of the 6 patients with erosive esophagitis, 5 had mild esophagitis and 1 moderate esophagitis. No patients with NERD showed histological signs of esophagitis. After 3 mo of therapy, 13/15 patients (86.7%, P<0.01) showed a complete recovery of DIS and disappearance of heartburn. Of the 2 patients treated for 3 more months, complete recovery of DIS and heartburn were achieved in one.
CONCLUSION: Three or 6 mo of omeprazole therapy led to a complete regression of the ultrastructural esophageal damage in 86.7% and in 93% of patients with DGER, NERD and ERD respectively. The ultrastructural recovery of the epithelium was accompanied by regression of heartburn in all cases.
- Citation: Calabrese C, Fabbri A, Bortolotti M, Cenacchi G, Carlo S, Zahlane D, Miglioli M, Febo GD. Effect of omeprazole on symptoms and ultrastructural esophageal damage in acid bile reflux. World J Gastroenterol 2005; 11(12): 1876-1880
- URL: https://www.wjgnet.com/1007-9327/full/v11/i12/1876.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i12.1876
Gastroesophageal reflux disease (GERD) is an important public health problem. Heartburn is a high common condition affecting up to 30% of the adults[1]. At endoscopy, approximately 60% of patients have a non-erosive reflux disease (NERD), 30% have erosive esophagitis and 10% have a metaplastic columnar-lined epithelium containing goblet cells, called Barrett’s esophagus[2-4]. Several studies have demonstrated that severity and frequency of GERD symptoms, typically heartburn and regurgitation, are not predictive of the presence of esophageal lesions[5]. These different esophageal responses to gastroesophageal reflux are poorly understood. Although the degree of reflux exposure may in part be responsible[6], there is considerable overlap between the magnitude of gastroesophageal reflux, assessed by 24-h ambulatory monitoring pH and bilimetry, and the type and degree of esophageal damage[7].
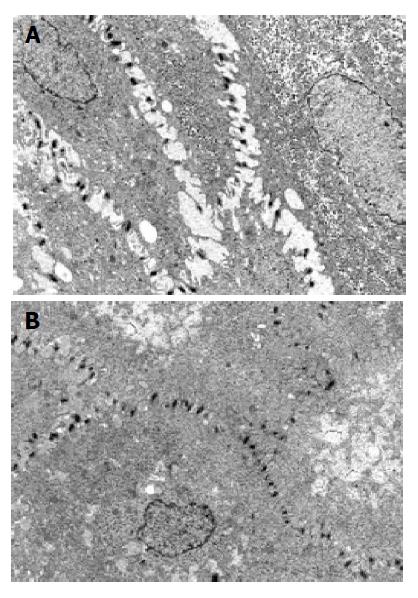
Recently, it has been recognized at transmission electron microscopy (TEM) a lesion in symptomatic patients with positive 24-h pH-monitoring, with or without positive bile testing[8]. This high sensitive and precocious sign of damage is the presence of dilated intercellular spaces (DIS) within the esophageal epithelium, either with erosive or non-erosive esophagitis[8,9]. The pathophysiological significance of DIS within esophageal epithelium in patients with erosive or non-erosive reflux disease remains partly unknown.
Esophageal exposure to gastric acid is considered the most important factor in the pathogenesis of GERD. Otherwise, the refluxate is often mixed with duodenal contents but the contribution of this phenomenon to esophageal mucosal damage is not well- known. For GERD, omeprazole is used in DGER but the effects on duodenogastric reflux have been reported in a few studies, which are not conclusive and lacking of deep morphological analysis[10-13].
Aim of this study is to value whether PPI treatment will induce, besides a regression of symptoms, an ultrastructural modification of the esophageal epithelium both in erosive and non erosive DGER.
Patients gave written informed consent to participate in the study, which was approved by the local research ethical committee in June 2001. The design of the study was single blinded for the histological and ultrastructural evaluation.
Fifteen patients (6 male) were affected by DGER as defined by typical symptoms (recurrent episodes of heartburn or acid regurgitation) and abnormal 24-h pH and bile testing parameters. Patients were excluded if they: had Barrett’s esophagus, were asymptomatic with positive pH-monitoring parameters, were undergoing NSAID or steroids therapy, or had peptic ulcerations, pyloric stenosis, gastric resection, or other severe organ diseases.
After calibration, a pH electrode and Bilitec fiber-optic probe were passed nasally and positioned 5 cm above of the lower esophageal sphincter. Subjects were sent home with instructions to keep a diary of symptoms, meal times, time of laying down for sleep and time of rising in the morning[14]. A diet was prescribed according to Barrett et al[15], with particular attention to substances that may interfere with bilirubin monitoring. Subjects returned the next day after 22 to 25-h of monitoring to have the probes removed and the diary reviewed. Several days later, an upper gastrointestinal endoscopy was performed and 6 biopsy specimens were taken from each patient. The biopsies were obtained from the lower 5 cm of esophagus from normal-appearing mucosa for histological and ultrastructural evaluation (3 biopsies respectively). After the initial assessment of ultrastructural damage, all patients were treated with omeprazole 40 mg once daily for 3 mo keeping a diary of symptoms (heartburn and regurgitation). The severity of symptoms were graded as follows: (1) mild, can be ignored (2) moderate, cannot be ignored but does not affect life-style; (3) severe, affects life-style; (4) very severe, markedly affects life-style. The score of frequency of symptoms was: (1) less than once month; (2) once a month; (3) once a week; (4) several times a week; (5) daily.
After 3 mo of therapy a further upper endoscopy was performed and 6 esophageal biopsies were taken.
In patients with persistence of heartburn and/or with an incomplete recovery of esophageal epithelium at TEM, a further endoscopy was performed after 3 more months of treatment with omeprazole 40 mg once daily.
Prolonged pH-monitoring esophageal study was performed using an antimony electrode. The pH electrode (Synectics Med., Stockholm, Sweden) was calibrated at 37 °C in pH 7.01 and pH 1.07 buffer solution (Fisher Scientific, Fairlawn, NJ) before and after completing each study. An AgCl reference electrode was placed on the anterior chest. The pH electrode was connected to a portable digital data recorder (Flexilog 2000, Oakfield Instruments LTD, Witney, UK) that stored pH data every 4 s for up to 24 h. At the conclusion of the study, information in the data recorders was downloaded into a PC for analysis using a Flexisoft III computer program (Oakfield Instruments LTD, UK). According to Johnson’s methodology[16], gastroesophageal reflux is considered to be for every drop in the intraesophageal pH to less than 4.0 for at least 20 s.
Prolonged esophageal monitoring of DGER was performed using a fiber-optic sensor, Bilitec 2000 (Medtronic, Düsseldorf, Germany). The system consists of a miniaturized fiber-optic probe that carries light signals into the esophagus and back to an optoelectronic system via a plastic fiber-optic bundle. Two light-emitting diodes (at 470 and 565 nm) represent the source for the measurement of bilirubin. The portable photodiode system converts the transmitted light into an electrical signal. After amplification, the signal is processed by an integrate microcomputer, which calculates the difference of the absorbances at 470 nm and 565 nm. This value is directly proportional to the bilirubin concentration in the sample under investigation. After conclusion of the study all data were downloaded into a PC for analysis using Gastrosoft 2000 (EsopHgram, Gastrosoft, Irving, TX, USA), which calculates the average value of the absorbance between two successively sampled values in order to reduce noise levels in the signal. The DGER data were calculated when the percentage time bilirubin absorbance level was ≥0.14 and analyzed separately for total time, as well as, upright and supine periods. A value ≥0.14 was chosen because values less than this number represent scattering due to suspended particles and mucus present in the gastric contents[14]. Acid and DGER were considered to be temporally related if they occurred within one minute during the 24-h study period.
All subjects underwent an upper GI endoscopy (video gastroscope Olympus GIF V2, Hamburg, Germany) after sedation by IV administration of midazolam (2, 5 mg). Six biopsies were obtained from the lower 5 cm of esophagus from areas of macroscopically non-eroded esophageal mucosa. The presence of esophagitis was noted and graded according to the Los Angeles Classification[17].
Three specimens from each patient were fixed in formalin immediately after endoscopy and then embedded in paraffin wax. Serial sections of 4 um thicknesses were cut and stained with hematoxylin-eosin. Esophagitis was identified and graded according to the Ismail-Beigi classification[18].
Three specimens from each patient were fixed in glutaraldehyde, rinsed and processed for TEM. The specimens were post-fixed in 1% buffered osmium tetroxide. They were then dehydrated through a graded alcohol series and embedded in Araldite. Blocks were trimmed and ultra-thin sections on copper grids were post-stained with uranyl acetate and lead citrate. Each specimen was analyzed by TEM (Philips 410, Eindhoven, Netherlands) and then photographed at an accelerating voltage of 80 kV. Photographs of at least 10 significant fields were magnified at 3500×.
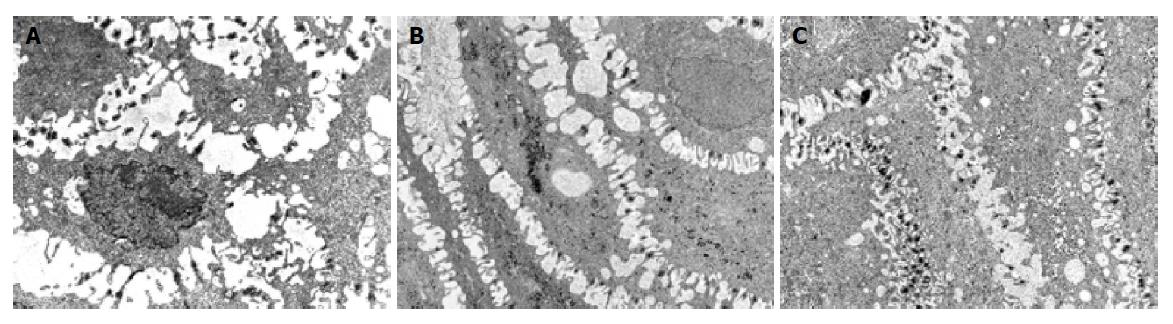
Ten TEM photomicrographs of biopsy specimens from each patient were obtained. In particular, the suprabasal layer of the esophageal mucosa was examined in each image. Photographs with an internal scale marker were digitized and then each field was evaluated using EndoxPro System (Casti imaging, Medra-Venice, Italy). At least 10 randomly selected perpendicular transects to adjacent membranes were drawn and measured in each image for a total of 100 measurements in each case. Every transect was drawn at a distance not closer than 1 μm. A value of mean score of DIS of 0.74 μm was considered a cut-off score for damage[8].
Measurements obtained were used to calculate mean DIS scores and mean scores of maximum DIS for each subject and for all cases as a whole. Statistical significance was determined using Student’s t-tests for paired and unpaired samples. Scores are reported as mean±SD. The results of treatment were compared by χ2 test for comparison of proportions with a 95%CI. All statistical analyses were two-tailed, and significance was accepted at a P-value < 0.05. Data were analyzed with SPSS software.
Fifteen patients (mean age 44.4±10.96; 6 male) had DGER as defined by typical symptoms (recurrent episodes of heartburn or acid regurgitation) and pathological 24-h pH- and bilimetry. Of these, 6 had erosive esophagitis (mean age 44.3±9.69; 3 men), whereas 9 had a NERD (mean age 40.8±12.97; 3 men). Twelve patients had hiatal hernia.
At histology, of the 6 patients with erosive esophagitis, 3 had mild esophagitis, 2 had moderate esophagitis and 1 had normal histological pattern. No patients with NERD showed signs of esophagitis at histology (Table 1).
| NERD | ERD | |
| Number | 9 | 6 |
| Sex (M/F) | 3/6 | 3/3 |
| Mean age ± SD (range) | 40.8±12.97 (28-64) | 44.3±9.69 (28-53) |
| Macroscopic findings | ||
| Hiatal hernia | 8 | 4 |
| Grade 0 | 9 | 0 |
| Grade A | 0 | 0 |
| Grade B | 0 | 5 |
| Grade C | 0 | 1 |
| Grade D | 0 | 0 |
| Histology | ||
| Normal | 9 | 0 |
| Mild | 0 | 5 |
| Moderate | 0 | 1 |
| Severe | 0 | 0 |
At endoscopy and at histology, the 6 patients with erosive esophagitis showed the complete healing after 3 mo of therapy.
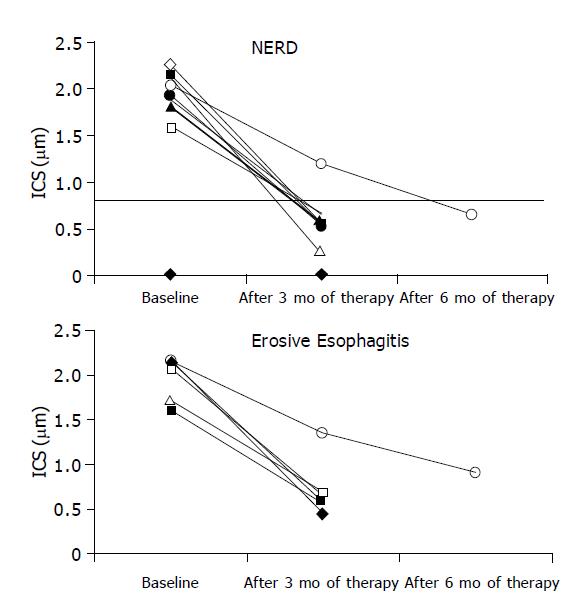
At TEM, after 3 mo of therapy 13/15 patients (86.7%, CI 85.73-88%, χ2 = 8.067, P<0.005) showed a complete recovery of the esophageal epithelium (Figure 1) accompanied by resolution of heartburn. In particular, 5/6 with erosive esophagitis (83.3%CI 82.7-84.56%) and 8/9 with NERD (88.9%CI 88.52-89.03%) reached this outcome (Figure 2).
Two patients, 1 with NERD and 1 with erosive esophagitis before treatment, required 3 further months of therapy because of an incomplete healing of the mucosa at TEM and persistence of symptoms. After this period, a complete recovery of esophageal mucosa and heartburn was achieved only in patient with NERD. In patient with erosive esophagitis, we observed a reduction of DIS (Figure 3) and the persistence of sporadic and moderate heartburn (Table 2). Hence, 14 of 15 patients (93.3%CI 92.48-94.56%, χ2 = 11.26, P<0.001) (Figure 2) achieved complete recovery of DIS and the resolution of heartburn.
| Endoscopy (L. A.) | Mean value of DIS ± SD (mm) | Frequency of symptoms | Severity of symptoms | |
| Patient 1 (female) | ||||
| Baseline | 0 | 2.14±0.81 | 5 | 4 |
| After 3 mo | 0 | 1.21±0.41 | 1 | 1 |
| After 3 further mo | 0 | 0.51±0.08 | 0 | 0 |
| Patient 2 (female) | ||||
| Baseline | C | 2.61±1.01 | 5 | 4 |
| After 3 mo | 0 | 1.15±0.42 | 3 | 2 |
| After 3 further mo | 0 | 0.92±0.12 | 1 | 1 |
Patients affected by GERD mostly require a potent suppression of gastric acid secretion, to obtain beneficial effects into the esophagus and usually this suppression must be greater to control acid peptic injury to esophagus as opposed to that of stomach[19]. It is supposed that the reasons for this phenomenon are related to specific differences between the nature of gastroduodenal and esophageal epithelial defenses. In particular, it is reported, for the esophageal epithelium: the lack of mucus, bicarbonate, prostaglandin secretion by surface epithelial cells and the low capacity to rapidly heal erosions by the process of epithelial restitution[19].
Using TEM, it has been demonstrated in GERD and in DGER that the most precocious and sensitive morphological feature of mucosal damage is DIS[8]. Recently, it has been verified that this marker is reversible after treatment with PPI in GERD[20]. In other words, after treatment, it seems that the mucosa restore the fence-like barrier to the acid attack partly repairing the dilation of intercellular space and in this way patients become asymptomatic[20]. This is a further confirmation that intercellular junctional space is part of the defense of the mucosa and its related resistance and that of acid, alone or mixed bile reflux, may damage it and determine a greater permeability for refluxate. What we do not know is whether PPI will determine the same effect in DGER.
It has been observed that DGER, without acid, does not play a major role in producing symptoms or lesions, while mixed DGER, which occur simultaneously in the majority of the patient with reflux disease, determines an increase in severity across GERD spectrum[6,21].
Otherwise, the effect of PPI on DGER, even if it is a field of interest, is reported only in few, contrasting, studies. In particular, in these studies treatment is related to the effect on the amount of total bilirubin esophageal exposure or to symptoms and endoscopic lesions[10-13]. Moreover, it is unknown the effective response to PPI in DGER, as it regards the morphological damage of the mucosa.
In our study, we analyzed at TEM several esophageal biopsies taken during endoscopy performed on 12 subjects with mixed reflux. Patients underwent an upper endoscopy and, irrespective of the presence of erosive esophagitis or normal appearing mucosa, were treated with omeprazole 40 mg daily for three months. After this period a further endoscopy with biopsies was performed and symptoms investigated. Our data support the following considerations.
At first, 86.6% of patients presented a complete resolution of symptoms after 3 mo of therapy. Two subjects, 1 with NERD and 1 with erosive esophagitis, required three more months of treatment because of persistent heartburn. At the end of this period one of them became asymptomatic while the other, with erosive esophagitis, complained of persisting symptoms but not more macroscopic or histologic signs of esophagitis[22-25]. It was noted that the histological pattern became normal after the first period of treatment in all subjects studied.
At TEM we observed complete recovery of DIS in 86.6% of patients after 3 mo of therapy and in 93.3% of patients after 6 mo. No significant differences between NERD and erosive esophagitis were seen. In addition, the ultrastructural healing of the esophageal mucosa was in all cases accompanied with complete resolution of the esophageal symptoms. The patient, still symptomatic after 6 mo of treatment, showed the persistence of DIS.
Similar data has been reported in our recent study concerning patients affected by GERD in which complete recovery of DIS was achieved in 92.1% and in 97.4% of cases after 3 and 6 mo of omeprazole 40 mg daily respectively[20].
In summary, this is the first demonstration that a long-term treatment with omeprazole may induce a complete healing of mucosal damage at TEM both in erosive and in NERD patients also with DGER. In this way, it seems that the presence of bile in refluxate does not significantly affect the response to therapy in our subset of patients.
Data concerning DGER and treatment are scanty and studies have been conducted with a small series of patients. Even if this could explain the heterogeneous results reported to date, we believe that beside differences among patients in the esophageal refluxate, motor activity, or other demographic and morphological features, potential additional factors such as the epithelial defense of the mucosa and its resistance may play a role in this contest that should be considered.
| 1. | Spechler SJ. Epidemiology and natural history of gastro-oesophageal reflux disease. Digestion. 1992;51 Suppl 1:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 267] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 2. | Heading RC. Epidemiology of oesophageal reflux disease. Scand J Gastroenterol Suppl. 1989;168:33-37. [PubMed] |
| 3. | Winters C, Spurling TJ, Chobanian SJ, Curtis DJ, Esposito RL, Hacker JF, Johnson DA, Cruess DF, Cotelingam JD, Gurney MS. Barrett's esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology. 1987;92:118-124. [PubMed] |
| 4. | Wienbeck M, Barnert J. Epidemiology of reflux disease and reflux esophagitis. Scand J Gastroenterol Suppl. 1989;156:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 126] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Galmiche JP, Barthelemy P, Hamelin B. Treating the symptoms of gastro-oesophageal reflux disease: a double-blind comparison of omeprazole and cisapride. Aliment Pharmacol Ther. 1997;11:765-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Vaezi MF, Richter JE. Role of acid and duodenogastroesophageal reflux in gastroesophageal reflux disease. Gastroenterology. 1996;111:1192-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 342] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 7. | Neumann CS, Iqbal TH, Cooper BT. Long term continuous omeprazole treatment of patients with Barrett's oesophagus. Aliment Pharmacol Ther. 1995;9:451-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 57] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Calabrese C, Fabbri A, Bortolotti M, Cenacchi G, Areni A, Scialpi C, Miglioli M, Di Febo G. Dilated intercellular spaces as a marker of oesophageal damage: comparative results in gastro-oesophageal reflux disease with or without bile reflux. Aliment Pharmacol Ther. 2003;18:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Tobey NA, Carson JL, Alkiek RA, Orlando RC. Dilated intercellular spaces: a morphological feature of acid reflux--damaged human esophageal epithelium. Gastroenterology. 1996;111:1200-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 332] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 10. | Champion G, Richter JE, Vaezi MF, Singh S, Alexander R. Duodenogastroesophageal reflux: relationship to pH and importance in Barrett's esophagus. Gastroenterology. 1994;107:747-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 276] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 11. | Marshall RE, Anggiansah A, Manifold DK, Owen WA, Owen WJ. Effect of omeprazole 20 mg twice daily on duodenogastric and gastro-oesophageal bile reflux in Barrett's oesophagus. Gut. 1998;43:603-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Ciamarra P, Sarnelli G, Flavia Savarese M, Russo L, Budillon G, Cuomo R. Aggressive acid suppression decreases not only acid but also bile reflux in gastro-oesophageal reflux disease. Dig Liv Dis. 2004;106. |
| 13. | Koek GH, Sifrim D, Lerut T, Janssens J, Tack J. Effect of the GABA(B) agonist baclofen in patients with symptoms and duodeno-gastro-oesophageal reflux refractory to proton pump inhibitors. Gut. 2003;52:1397-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 205] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 14. | Bechi P, Pucciani F, Baldini F, Cosi F, Falciai R, Mazzanti R, Castagnoli A, Passeri A, Boscherini S. Long-term ambulatory enterogastric reflux monitoring. Validation of a new fiberoptic technique. Dig Dis Sci. 1993;38:1297-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 134] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Barrett MW, Myers JC, Watson DI, Jamieson GG. Dietary interference with the use of Bilitec to assess bile reflux. Dis Esophagus. 1999;12:60-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Johnson LF, Demeester TR. Twenty-four-hour pH monitoring of the distal esophagus. A quantitative measure of gastroesophageal reflux. Am J Gastroenterol. 1974;62:325-332. [PubMed] |
| 17. | Armstrong D, Bennett JR, Blum AL, Dent J, De Dombal FT, Galmiche JP, Lundell L, Margulies M, Richter JE, Spechler SJ. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology. 1996;111:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 790] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 18. | Ismail-Beigi F, Horton PF, Pope CE. Histological consequences of gastroesophageal reflux in man. Gastroenterology. 1970;58:163-174. [PubMed] |
| 19. | Orlando RC. Why is the high grade inhibition of gastric acid secretion afforded by proton pump inhibitors often required for healing of reflux esophagitis? An epithelial perspective. Am J Gastroenterol. 1996;91:1692-1696. [PubMed] |
| 20. | Calabrese C, Bortolotti M, Fabbri A, Areni A, Cenacchi G, Scialpi C, Miglioli M, Di Febo G. Reversibility of GERD ultrastructural alterations and relief of symptoms after omeprazole treatment. Am J Gastroenterol. 2005;100:537-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 156] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | Koek GH, Tack J, Sifrim D, Lerut T, Janssens J. The role of acid and duodenal gastroesophageal reflux in symptomatic GERD. Am J Gastroenterol. 2001;96:2033-2040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Lind T, Cederberg C, Ekenved G, Haglund U, Olbe L. Effect of omeprazole--a gastric proton pump inhibitor--on pentagastrin stimulated acid secretion in man. Gut. 1983;24:270-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 397] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 23. | Festen HP, Tuynman HA, Défize J, Pals G, Frants RR, Straub JP, Meuwissen SG. Effect of single and repeated doses of oral omeprazole on gastric acid and pepsin secretion and fasting serum gastrin and serum pepsinogen I levels. Dig Dis Sci. 1986;31:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Lillemoe KD, Johnson LF, Harmon JW. Role of the components of the gastroduodenal contents in experimental acid esophagitis. Surgery. 1982;92:276-284. [PubMed] |
| 25. | Harmon JW, Johnson LF, Maydonovitch CL. Effects of acid and bile salts on the rabbit esophageal mucosa. Dig Dis Sci. 1981;26:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 91] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
Science Editor Guo SY Language Editor Elsevier HK