Published online Jan 7, 2005. doi: 10.3748/wjg.v11.i1.132
Revised: December 15, 2003
Accepted: February 1, 2004
Published online: January 7, 2005
AIM: To investigate the protective effect of ginkgo biloba extract (GBE) on livers of aged rats and the associated mechanisms.
METHODS: Two-mo- and 20-mo-old rats were treated with GBE/saline for 3 mo. Liver tissue samples from 5-mo-old rats treated with saline (group Y) and 23-mo-old rats treated with GBE (group E) or saline (group N) were used for histopathological examinations (hematoxylin-eosin and Masson staining, Lipofuscin staining-Schmorl staining) and determination of expression of tissue inhibitor-1 of metalloproteinase (TIMP-1) and the level of malondialdehyde (MDA), glutathione peroxidase (GPx) and superoxide dismutase (SOD). Blood samples were collected for determination of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL) and albumin.
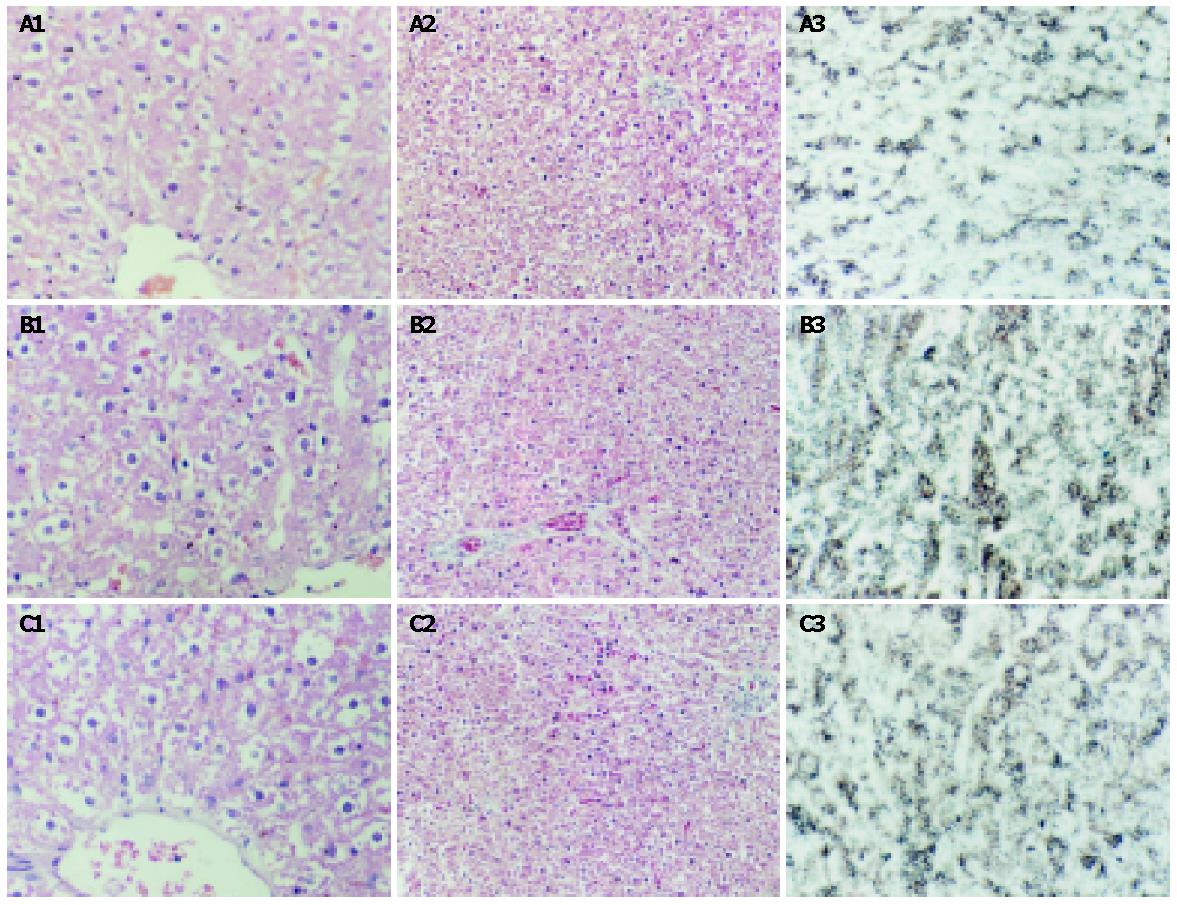
RESULTS: Microscopic studies with Masson staining revealed mild liver fibrosis in aged rats (group N), while the livers of aged rats receiving GBE (group E) showed amelioration in fibrosis (2.2±0.1 vs 2.8±0.1, P<0.01) and deposition of lipofuscin (33.7±5.3 vs 62.8±5.7, P<0.01). The expression of TIMP-1 and the level of liver MDA (1.0±0.1 vs 1.2±0.2, P<0.05) also decreased but the activity of GPx (97.1±15.3 vs 61.8±14.5, P<0.01) increased in group E. Compared with group Y, the level of liver MDA (0.8±0.1 vs 1.2±0.2, P<0.01), lipofuscin (32.4±6.0 vs 62.8±5.7, P<0.01) and TIMP-1 expression were increased, while the activity of GPx (103.2±17.6 vs 61.8±14.5, P<0.01) and SOD (16.7±4.4 vs 11.8±3.9, P<0.05) was decreased in group N. There was no difference in liver function among these three groups.
CONCLUSION: GBE has protective effects on aging liver. The possible mechanisms might be its antioxidant activity and inhibition of TIMP-1 expression.
- Citation: Huang SZ, Luo YJ, Wang L, Cai KY. Effect of ginkgo biloba extract on livers in aged rats. World J Gastroenterol 2005; 11(1): 132-135
- URL: https://www.wjgnet.com/1007-9327/full/v11/i1/132.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i1.132
Fibrosis is a hallmark of aging of various organs, including the heart and kidney, and reflects increased deposition of the physiological components of the extracellular matrix. Aging is also associated with variable degrees of fibrosis in liver[1]. Oxidative stress (OS) might represent a direct or indirect relevant pro-fibrogenic stimulus for hepatic stellate cells (HSCs)[2], as suggested by in vivo experimental studies in which administration of antioxidants prevents OS, lipid peroxidation and liver fibrosis[3-6]. Ginkgo biloba extract (GBE) is an extract from green leaves of the ginkgo biloba tree. GBE has been shown to have a SOD-like activity and hydroxyl radical scavenging activity[7-11]. Since GBE is known to exert protective influences against the action of free radicals, we hypothesized that the application of such extracts might prevent liver fibrosis in aged rats. To our knowledge, there lacks the information in literature about the capacity of GBE to prevent free radical formation and peroxidation in liver fibrosis in aged rats.
GBE was purchased from Hubei Wushi Pharmaceutical Company, China (No. 21003). The GBE and double-distilled water were mixed to form 0.1 mg/mL suspension. SOD, MDA and GPx kits were purchased from Nanjing Jiancheng Biological Technology Company, China. RNasin and 2000 bp molecular weight ladders were purchased from Huamei Biologic Technology Company, China. Moloney murine leukemia viral (MmuLV) reverse transcriptase was purchased from Promega Biotechnology Co. Ltd, USA. Deoxynucleotide triphosphates (dNTPs) were purchased from Takara Biotechnology Co. Ltd, USA. Trizol and Taq DNA polymerase were purchased from Biostar Co. Ltd, USA. Thermal cyclor was purchased from Biometra UNOII, USA. Light microscopes were purchased from Olympus Co. Ltd., Japan.
Six 2-mo-old (group Y) and twelve 20-mo-old (group N and group E) male inbred Wistar rats were purchased from the Experimental Animal Center of Wuhan University of Medical Sciences, China (20021220). Animals were fed standard rat chow with free access to tap water and received humane care in accordance with the animal care provisions, and were kept in temperature- and humidity-controlled animal quarters with a 12-h light-dark cycle. The rats were weighed daily. GBE 200 mg/(kg.d) was given orally to group E by gavage, whereas group N and group Y rats received only saline. The experiment lasted for 3 mo. At the end of experiment, the animals were anesthetized with ether and kept at a constant temperature of (37.0±0.5) °C. One blood sample was taken, centrifuged at 3000 r/min for 10 min, and the plasma was stored until use. Then the animals were exsanguinated and the liver was quickly washed in situ with ice-cold isotonic saline, removed and weighed, and each liver was divided into two portions, one was for histological study (immunohistochemical staining, H-E and Masson staining), the other was immediately frozen in liquid nitrogen. Serum levels of TBIL and albumin, and activity of ALT and AST were determined by standard hospital laboratory methods.
The level of MDA in liver tissue was determined according to the methods of Yagi and Sanz et al[2]. Hepatic SOD was assayed according to Misra and Fridovich[2]. GPx activity was examined according to Flohe and Gunsler[2]. Protein concentrations were measured by the method of Lowry using bovine serum albumin as standard.
Liver tissue sections were fixed in 100 mL/L formalin saline in phosphate buffer and processed in paraffin wax. Sections from blocks were stained with hematoxylin-eosin and Masson’s trithrome. The level of lipofuscin in liver tissue was determined by the methods of Schmorl[2]. Qualitative and quantitative histological analyses were performed blindly using light microscope and computer image analysis system. The image intensity was maintained at the same level throughout the study. The collagenous deposits were observed at 40× magnification in centrilobular field of the hepatic acinus for each sample, and in surrounding terminal hepatic veins. In order to avoid possible bias due to sampling of the individual fields, we examined at least 5 fields each containing a centrilobular vein and the same was done for quantity of hepatocytes at 400× magnification.
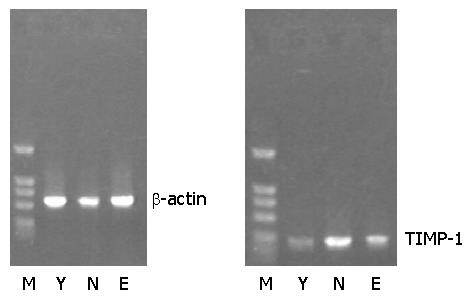
Total RNA was extracted using Trizol according to manufacturer’s directions, and reverse transcribed into cDNA. PCR was performed using the following primer pairs: β-actin, sense 5’-ATCATGTTTGAGACCTTCAACACC-3’ and antisense 5’-CATGGTGGTGCCGCCAGACAG-3’ (556 bp), TIMP-1[12], sense 5’-ACAGCTTTCTGCAACTCG-3’, and antisense 5’-CTATAGGTCTTTACGAAGGCC-3’ (335 bp). The annealing temperatures were 60 °C and 57 °C respectively. The amplified products were electrophoresed on 12 g/L agarose gel containing 0.5 μg/mL ethidium bromide and visualised under UV light.
Data were presented as mean±SD. Statistical evaluations were performed by using the Student’s t-test. χ2 test was used for histopathological parameters. P<0.05 was considered statistically significant.
There was no death of any rat during experiment. Liver and body weights (LW and BW, respectively) of rats are presented in Table 1. LW increased with age but was proportionally less than body weight, the ratio of LW to BW declined. The ratio increased after treatment with GBE.
| BW (g,before) | BW (g,after) | LW (g) | LW/BW (%) | |
| Y | 224.3±14.2d | 420.3±13.6d | 15.9±0.8d | 3.7±0.1d |
| N | 763.8±21.5b | 768.8±22.6b | 20.4±3.5b | 2.7±0.3b |
| E | 761.0±19.6b | 750.4±25.4b | 19.3±1.1b | 2.6±0.1b |
Histology of livers from the rats of group Y was normal. With Masson method, collagenous protein staining was distinctly blue. Light microscopy revealed mild fibrosis in liver sections and collagenous protein accumulation in portal and centrilobular areas in aged rats. Qualitative and quantitative histological analyses showed GBE markedly improved the degree of hepatic fibrosis in aging rats in group E, but liver sections taken from group N and group E had more collagenous deposits than those from group Y (Table 2, Figure 1). Hepatocytes were arranged disorderly in group N, and were larger in group Y. The lipofuscin was distributed intensively in hepatocytes of group N compared with group Y. It was distributed scarcely in hepatocytes of group E, less than that in hepatocytes of group N.
| Hepatocyte (×103) | Fibrotic area (%) | Lipofuscin (%) | |
| Y | 5.3±0.3d | 1.4±0.1d | 32.4±6.0d |
| N | 2.3±0.3b | 2.8±0.1b | 62.8±5.7b |
| E | 3.4±0.4bd | 2.2±0.1bd | 33.7±5.3d |
Serum activity of ALT and AST, liver concentration of MDA and activity of GPx and SOD are shown in Table 3. Liver MDA levels increased while activity of GPx and SOD decreased in aging rats. The condition was ameliorated after rats were treated with GBE, but there was a significant distinction between groups E and Y.
| ALT (U/L) | AST (U/L) | TBIL (g/L) | Albumin (g/L) | MDA nmol/g) | GPx (U/g) | SOD (U/mg) | |
| Y | 50.4±7.70 | 109.1±28.6 | 5.3±0.8 | 44.7±4.8 | 0.8±0.1d | 103.2±17.6d | 16.7±4.4c |
| N | 59.0±23.7 | 131.8±22.2 | 5.9±0.7 | 40.9±3.1 | 1.2±0.2b | 61.8±14.5b | 11.8±3.9a |
| E | 61.8±25.2 | 118.3±28.4 | 5.1±0.6 | 42.5±6.9 | 1.0±0.1bc | 97.1±15.3d | 14.1±4.7 |
RT-PCR analysis revealed a weak expression of TIMP-1 mRNA in groups Y and E, and an obvious expression of TIMP-1 mRNA in group N (Figure 2).
Aging is usually associated with increasing level of oxidation[12-13]. An imbalance between the formation and removal of reactive oxygen species (ROS) and the development of OS plays an important role in aging and age-associated diseases[14-16]. ROS alters proteins, carbohydrates, and lipids, and inactivates enzymes and transporters, damages DNA and the transcriptional machinery, and initiates the chain reactions that peroxidize polyunsaturated fatty acids in membrane phospholipids[17]. The normal liver is a well equipped organ in terms of either enzymatic or non-enzymatic antioxidants. At molecular level, growth factors, cytokines and chemokines, changes in extracellular matrix (ECM) organization and composition as well as reactive molecules induced by OS, play a pathogenetic role. OS-related molecules may act as mediators to modulate tissue and cellular events responsible for the progression of liver fibrosis[18,19]. Signs of OS and lipid peroxidation are concomitant with or preceding HSC activation and collagen deposition. Several studies indicate that the most frequent age-dependent changes are the reduction in organ mass, hepatocyte enlargement and degeneration, and the increase in individual mitochondrial volume and the decline in their number and bile duct proliferation[5].
MDA is a degradative byproduct of lipid peroxidation. MDA levels are utilized as an indicator of oxidative damage. But our study showed that the levels of MDA in 23-mo-old rats increased significantly as compared with 5-mo-old rats, which is in favor of a strong OS and enhanced ROS formation in senescent rats. Hepatic GPx and SOD activity were both 1.3-fold higher in 5-mo-old rats than in 23-mo-old rats. The total antioxidant capacity of liver cells is not sufficient to scavenge the ROS generated in senescent animals. The lower activity of SOD in aged rats may be a consequence of inhibitory effects due to excess ROS generation. According to currently available literature, the inconsistent results obtained from different studies might reflect variations in species, strain, sex, and experimental design[19]. Collagenous protein is frequently detectable in the parenchyma in aged rats, and mainly diffuses along the sinusoidal walls. Image analysis of Masson staining sections showed the accumulation of collagenous protein, accounting for 1.4% and 2.8% of the total area in 5-mo-old and 23-mo-old rats respectively. It indicates an increase in the collagenous protein accumulation with age, mainly within the portal tracts. The deposition of lipofuscin in hepatocytes also increased in 23-mo-old rats. Liver function tests remain normal in senescent individuals. The enhanced susceptibility of senescent animals is attributed to elevated levels of oxidative damage[16].
GBE is used as a standardized recipe preparation and contains two groups of major substances: flavonoid glycosides and terpenoids. GBE has been used therapeutically for centuries. Furthermore, GBE has the capability of inactivating oxo-ferryl radical species, which are more efficient oxidative agents than classical hydroxyl radicals[11]. These features of the natural antioxidant GBE bring many beneficial effects against free radical injuries. However, as a therapeutic agent, the role of GBE in liver fibrosis in aging rats needs to be further investigated.
The level of MDA in liver tissue was significantly decreased while the activity of GPx and SOD was increased in GBE-treated group. Our study confirmed that GBE could inhibit lipid peroxidation in liver tissue and protect the membrane protein from the polymerization induced by lipid peroxidation. In histopathological examination, fibrosis was found to be significantly decreased in GBE-treated group, suggesting that GBE prevents deposition of lipofuscin. Thus, GBE might be effective in blocking the development of liver fibrosis in aging liver by reducing the formation of lipid peroxidation.
The cellular and molecular events underlying fibrogenesis have been investigated, but so far few data are available about the changes in hepatic collagenous protein metabolism during aging available. ECM turnover is a vital step in the tissue remodeling that accompanies physiological and pathological processes[20,21]. Many newly synthesized collagenous proteins are immediately degraded, the extent of this process is primarily regulated by metalloproteinases (MMPs), whose activity under physiological conditions is precisely down-regulated by TIMPs[22]. Lasting perturbations of this step could lead to liver fibrosis[23]. In our study, we found the increased expression of TIMP-1 mRNA in aging rats, but the expression of TIMP-1 mRNA was declined in GBE-treated aged rats.
In conclusion, GBE seems to be effective in preventing the development of fibrosis in aged rats. Its protective effect may be due to its capacity of inhibiting lipid peroxidation and expression of TIMP-1 as well as enhancing hepatocellular proliferation.
| 1. | Sun WB, Han BL, Peng ZM, Li K, Ji Q, Chen J, Wang HZ, Ma RL. Effect of aging on cytoskeleton system of Kupffer cell and its phagocytic capacity. World J Gastroenterol. 1998;4:77-79. [PubMed] |
| 2. | Sanz N, Díez-Fernández C, Alvarez AM, Fernández-Simón L, Cascales M. Age-related changes on parameters of experimentally-induced liver injury and regeneration. Toxicol Appl Pharmacol. 1999;154:40-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Sakai Y, Zhong R, Garcia B, Zhu L, Wall WJ. Assessment of the longevity of the liver using a rat transplant model. Hepatology. 1997;25:421-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Schmucker DL. Aging and the liver: an update. J Gerontol A Biol Sci Med Sci. 1998;53:B315-B320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 120] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Chao C, Youssef J, Rezaiekhaleigh M, Birnbaum LS, Badr M. Senescence-associated decline in hepatic peroxisomal enzyme activities corresponds with diminished levels of retinoid X receptor alpha, but not peroxisome proliferator-activated receptor alpha. Mech Ageing Dev. 2002;123:1469-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Arthur MJ. Matrix degradation in the liver. Semin Liver Dis. 1990;10:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Wu Z, Smith JV, Paramasivam V, Butko P, Khan I, Cypser JR, Luo Y. Ginkgo biloba extract EGb 761 increases stress resistance and extends life span of Caenorhabditis elegans. Cell Mol Biol (Noisy-le-grand). 2002;48:725-731. [PubMed] |
| 8. | Gohil K, Packer L. Bioflavonoid-rich botanical extracts show antioxidant and gene regulatory activity. Ann N Y Acad Sci. 2002;957:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Tang Y, Lou F, Wang J, Li Y, Zhuang S. Coumaroyl flavonol glycosides from the leaves of Ginkgo biloba. Phytochemistry. 2001;58:1251-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Mazzanti G, Mascellino MT, Battinelli L, Coluccia D, Manganaro M, Saso L. Antimicrobial investigation of semipurified fractions of Ginkgo biloba leaves. J Ethnopharmacol. 2000;71:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Schindowski K, Leutner S, Kressmann S, Eckert A, Müller WE. Age-related increase of oxidative stress-induced apoptosis in mice prevention by Ginkgo biloba extract (EGb761). J Neural Transm. 2001;108:969-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | McCarroll JA, Phillips PA, Kumar RK, Park S, Pirola RC, Wilson JS, Apte MV. Pancreatic stellate cell migration: role of the phosphatidylinositol 3-kinase(PI3-kinase) pathway. Biochem Pharmacol. 2004;67:1215-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med. 2000;28:463-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 932] [Cited by in RCA: 867] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 14. | Rikans LE, Hornbrook KR. Lipid peroxidation, antioxidant protection and aging. Biochim Biophys Acta. 1997;1362:116-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 260] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Johnson FB, Sinclair DA, Guarente L. Molecular biology of aging. Cell. 1999;96:291-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 337] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 16. | Palomero J, Galán AI, Muñoz ME, Tuñón MJ, González-Gallego J, Jiménez R. Effects of aging on the susceptibility to the toxic effects of cyclosporin A in rats. Changes in liver glutathione and antioxidant enzymes. Free Radic Biol Med. 2001;30:836-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 1607] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 18. | Poli G. Pathogenesis of liver fibrosis: role of oxidative stress. Mol Aspects Med. 2000;21:49-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 458] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 19. | Vendemiale G, Grattagliano I, Altomare E. An update on the role of free radicals and antioxidant defense in human disease. Int J Clin Lab Res. 1999;29:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 171] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Parola M, Robino G. Oxidative stress-related molecules and liver fibrosis. J Hepatol. 2001;35:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 567] [Article Influence: 22.7] [Reference Citation Analysis (1)] |
| 21. | Guo MZ, Li XS, Xu HR, Mei ZC, Shen W, Ye XF. Rhein inhibits liver fibrosis induced by carbon tetrachloride in rats. Acta Pharmacol Sin. 2002;23:739-744. [PubMed] |
| 22. | Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1301] [Cited by in RCA: 1339] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 23. | Gassó M, Rubio M, Varela G, Cabré M, Caballería J, Alonso E, Deulofem R, Camps J, Giménez A, Pajares M. Effects of S-adenosylmethionine on lipid peroxidation and liver fibrogenesis in carbon tetrachloride-induced cirrhosis. J Hepatol. 1996;25:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
Edited by Kumar M and Zhu LH