Published online Jan 7, 2005. doi: 10.3748/wjg.v11.i1.114
Revised: February 25, 2004
Accepted: March 2, 2004
Published online: January 7, 2005
AIM: To construct a recombinant attenuated Salmonella typhimurium DNA vaccine carrying Helicobacter pylori hpaA gene and to detect its immunogenicity.
METHODS: Genomic DNA of the standard H pylori strain 17 874 was isolated as the template, hpaA gene fragment was amplified by polymerase chain reaction (PCR) and cloned into pUCmT vector. DNA sequence of the amplified hpaA gene was assayed, then cloned into the eukaryotic expression vector pIRES through enzyme digestion and ligation reactions. The recombinant plasmid was used to transform competent Escherichia coli DH5α, and the positive clones were screened by PCR and restriction enzyme digestion. Then, the recombinant pIRES-hpaA was used to transform LB5000 and the recombinant plasmid isolated from LB5000 was finally used to transform SL7207. After that, the recombinant strain was grown in vitro repeatedly. In order to identify the immunogenicity of the vaccine in vitro, the recombinant pIRES-hpaA was transfected to COS-7 cells using LipofectamineTM2000, the immunogenicity of expressed HpaA protein was detected with SDS-PAGE and Western blot.
RESULTS: The 750-base pair hpaA gene fragment was amplified from the genomic DNA and was consistent with the sequence of H pylori hpaA by sequence analysis. It was confirmed by PCR and restriction enzyme digestion that H pylori hpaA gene was inserted into the eukaryotic expression vector pIRES and a stable recombinant live attenuated Salmonella typhimurium DNA vaccine carrying H pylori hpaA gene was successfully constructed and the specific strip of HpaA expressed by pIRES-hpaA was detected through Western blot.
CONCLUSION: The recombinant attenuated Salmonella typhimurium DNA vaccine strain expressing HpaA protein with immunogenicity can be constructed and it may be helpful for further investigating the immune action of DNA vaccine in vivo.
-
Citation: Xu C, Li ZS, Du YQ, Tu ZX, Gong YF, Jin J, Wu HY, Xu GM. Construction of a recombinant attenuated
Salmonella typhimurium DNA vaccine carryingHelicobacter pylori hpaA. World J Gastroenterol 2005; 11(1): 114-117 - URL: https://www.wjgnet.com/1007-9327/full/v11/i1/114.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i1.114
Helicobacter pylori is a Gram-negative microaerophillic bacterium which clones human gastric epithelium. Infection of H pylori is strongly associated with chronic gastritis, peptic ulcer or gastric adenocarcinoma and mucosa-associated lymphoid tissue lymphoma[1-4]. More than 50% of the population worldwide is infected with H pylori. The current standard treatment for it consists of antibiotics in combination with proton pump inhibitors[5-7]. Because of the emergence of antibiotic-resistant strains, vaccination of humans against H pylori infection is an effective and economical approach to the control of this pathogen.
Recently, DNA vaccine has been demonstrated to induce both humoral and cellular immunity and it is becoming a promising treatment for viral, bacterial and parasitic pathogens. Protective immunity against HIV, influenza virus, rabies virus, malaria and tuberculosis has been shown in animal models[8-12].
In this study, we constructed a recombinant live attenuated Salmonella typhimurium DNA vaccine carrying H pylori hpaA gene, and identified its immunogenicity in COS-7 cells in vitro.
Attenuated S typhimura LB5000 and SL7207 were kindly provided by Professor Bruce Stocker of Stanford University, USA. They were cultured in Amp (-) LB medium. COS-7 cell line was provided by the Department of Immunology, Secondary Military Medical University of China. E.coli DH5α used for cloning experiments was grown in LB medium containing 50 mg ampicillin per liter. Standard H pylori strain CCUG17874 (NCTC11638) was kindly provided by the Italian IRIS Research Center and cultured on H pylori-selective agar plates with 10% defibrillated sheep blood and antibiotics (Merck Company, Germany) at 37 °C under microaerophilic conditions with 50 ml/L O2, 10 mL/L CO2 and 85% N2. Vector pIRES was purchased from Clontech, USA.
H pylori strains were collected from the agar plates in PBS, then genomic DNA was extracted as previously described using CTAB. According to the complete DNA sequence of H pylori published and multiple clone sites of pIRES, the primers to amplify hpaA containing EcoRI site in P1 and MluI site in P2 were designed: P1: 5’ GAATTCCACCATGAAAAAAGGTAGTTTGGC3’, P2: 5’ ACGCGTCTACTTTCGTTTTTTCATTTCAC 3’. Amplication was done in a total volume of 50 μL under conditions: at 94 °C for 5 min, then 30 cycles at 94 °C for 45 s, at 55 °C for 45 s and at 72 °C for 1 min, followed by 5 min at 72 °C. The PCR products were analyzed on 1.2% agarose gels stained with ethidium bromide.
PCR products were separated using a QIAquick gel extraction kit (QIAGEN, CA, USA). Purified hpaA DNA fragments were subcloned into TA cloning vector pUCmT (Takara, Dalian, China), and then the sequence of hpaA was analyzed using an automatic sequencer.
Fragments of EcoRI and Mlu I-digested pUCmT-hpaA were inserted into the EcoRI/Mlu I site of eukaryotic expression vector pIRES, through a series of enzyme digestion and ligation reactions. Then the recombinant pIRES-hpaA was confirmed by PCR and restriction enzyme digestion.
Recombinant pIRES-hpaA was used to transform attenuated Salmonella typhimurium LB5000 with calcium chloride, then the recombinant plasmid was extracted to transform the final host strain SL7207 using electroporation.The attenuated Salmonella typhimurium SL7207 carrying hpaA gene was cultured in LB medium to 80 generations. The recombinant plasmid in transformed SL7207 were isolated from every 10 generations and identified by restriction enzymes and PCR.
To detect the protein expressed by recombinant pIRES-hpaA, pIRES-hpaA was transfected into COS-7 cells. COS-7 cell line was cultured at 37 °C, 5 mL/L CO2 in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS (Gibco-BRL, UK), 100 U/mL penicillin and 100 μg/mL streptomycin, 15 mmol/L HEPES, and 2 mmol/L L-glutamine. Twenty four hours before transfection, 5×105 COS7 cells were seeded into six-well plates, and the mixture of pIRES-hpaA and LipofectamineTM2000 (Invitrogen, USA) were added to the cells. Forty-eight hours after transfection, cells were washed with PBS, and protein extraction reagent (Pierce, USA) was added. After shaking for 5 min, the lysate was collected and centrifuged at 12000 g for 5 min at 4 °C. Supernatant containing the proteins was maintained at -80 °C until later use.
Supernatant containing the proteins was determined by electrophoretical analysis in a 12% polyacrylamide gel, subsequently electrotransferred onto nitrocellulose membranes (Bio-Rad, Germany), nonspecific binding sites were blocked with 2% bovine serum albumin ( BSA), then rabbit anti-H pylori and peroxidase-labeled anti-rabbit immunoglobulin G (IgG) were added (DAKO, Denmark). The antigens were visualized by chemiluminescence (Bio-Rad, Germany) according to the manufacturer’s instructions.
PCR products of hpaA were cloned into TA cloning vector pUCmT. The sequence of amplification fragment was consistent with that of H pylori hpaA published in the gene bank.
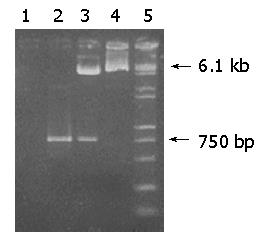
After pUCmT-hpaA and pIRES were digested by both EcoRI and Mlu I, a 750-bp fragment of hpaA was directly cloned into EcoRI/Mlu I site of pIRES, resulting in a recombinant plasmid pIRES-hpaA. pIRES-hpaA was digested by both EcoRI and Mlu I. P1 and P2 were used as primers to amplify hpaA from pIRES-hpaA, and the products analyzed on agarose gel (Figure 1) showed that the recombinant plasmid contained the objective gene hpaA.
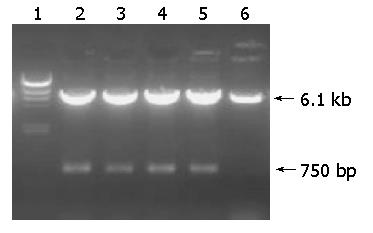
After transformed by pIRES-hpaA, the recombinant plasmid extracted from LB5000 was used to transform SL7207. Plasmid stability was essential to assure the stable expression of antigens encoded by genes which were cloned into the plasmid. Therefore, SL7207 carrying plasmid pIRES-hpaA was grown in vitro up to 80 generations to examine the plasmid stability. The 750-bp objective fragment could be seen on the map of agarose gel of the PCR products and the products of EcoRI and Mlu I-digested recombinant plasmid were isolated from transformed SL7207 (Figures 2 and 3).
Identification of pIRES-hpaA in vitro expression was carried out. The lysate of COS-7 cells transfected by pIRES-hpaA was analyzed by Western blotting. It revealed the immunoreactive band of 30-kD corresponded to HpaA protein, but the control transfected with pIRES had no specific band (Figure 4).
DNA vaccine is a novel vaccine. It has been widely used in laboratory animals and non-human primates to induce humoral and cellular immune responses. Clinical trials have shown that DNA vaccine is safe and well tolerated. Moreover, some reports have indicated that it could produce long-lasting immunity. The vaccine is a recombinant plasmid with heat stability. It can be used not only for protection but also for treatment in the presence of targeted infectious pathogens[13-15].
However, at present researches of H pylori vaccine mostly focus on protein vaccine, including H pylori whole-cell sonicate or one of the recombinant proteins of H pylori as the antigen of the vaccine in combination with mucosal adjuvants such as cholera toxin or heat-labile toxin of enterotoxigenic E.coli[16-18]. The manufacture of such vaccines is complicated, and some mucosal adjuvants have gastrointestinal toxicity. It was reported that mucosal immunization with Helicobacter heilmannii urease B or H pylori urease, given nasally with cholera toxin, could protect BALB/c mice against Helicobacter heilmannii infection and significantly reduce the pre-existing infection. However, immunization could aggravate gastric corpus atrophy[19].
H pylori adhesin A (HpaA) belonging to a group of outer membrane proteins of H pylori has been described as an adherence factor for blood cells and plays an important role in adhesion of microbes[20,21]. HpaA could mediate binding to sialic acid, a putative neuraminyllactose-binding hemagglutinin. HpaA is a highly conserved protein among H pylori clinical isolates and immunogenic in humans[22,23]. Therefore, HpaA is an ideal antigen candidate for H pylori vaccine.
It has been shown that live attenuated bacteria carrier including attenuated strains of Salmonella and Shigella in vitro could deliver DNA vaccines to human cells. Bacterial DNA vaccine delivery has also been demonstrated in vivo in several experimental animal models of infectious diseases and tumors. They allow vaccination via mucosal surfaces and specific targeting to professional antigen-presenting cells in mucosa-associated lymphoid tissue[24-26].
In this study, we constructed a live recombinant attenuated Salmonella typhimurium DNA vaccine strain expressing HpaA protein. First, the complete hpaA gene fragment was amplified from genomic DNA of H pylori; then sequence analysis was performed after it was cloned into the TA cloning vector pUCmT. Subsequently, purified hpa A was cloned to eukaryotic expression vector pIRES. Both the enzyme digestion and PCR confirmed the successful construction of recombinant plasmid pIRES-hpaA. Recombinant attenuated Salmonella typhimurium carrying H pylori hpaA gene was successfully constructed after pIRES-hpaA was first used to transform LB5000 and SL7207. The stability of the protective antigen is very important for a vaccine; we assessed the stability of the recombinant plasmid in vitro. It is confirmed by PCR and restriction enzyme that pIRES-hpaA is present in all the transformed strains of SL7207 up to the 80th generation, which reveals the stability of the recombinant plasmid in the bacterium.
It is also demonstrated in vitro in our present study that the COS-7 cellls transfected by pIRES-hpaA could express the specific protein of 30 kD, but the COS-7 cells transfected by pIRES could not express the protein. The pIRES-hpaA DNA vaccine could express the specific HpaA protein which can react with anti-H pylori.
Recombinant attenuated Salmonella typhimurium DNA vaccine carrying H pylori hpaA gene can express HpaA protein with immunogenicity. Further study is needed to explore its protective and therapeutic effect on animal models in vivo.
| 1. | Moss SF, Sood S. Helicobacter pylori. Curr Opin Infect Dis. 2003;16:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Al-Akwaa AM, Siddiqui N, Al-Mofleh IA. Primary gastric lymphoma. World J Gastroenterol. 2004;10:5-11. [PubMed] |
| 3. | Takahashi S. Long-term Helicobacter pylori infection and the development of atrophic gastritis and gastric cancer in Japan. J Gastroenterol. 2002;37 Suppl 13:24-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Isakov V, Malfertheiner P. Helicobacter pylori and nonmalignant diseases. Helicobacter. 2003;8 Suppl 1:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Suzuki H, Masaoka T, Nomura S, Hoshino Y, Kurabayashi K, Minegishi Y, Suzuki M, Ishii H. Current consensus on the diagnosis and treatment of H. pylori-associated gastroduodenal disease. Keio J Med. 2003;52:163-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Perri F, Qasim A, Marras L, O'Morain C. Treatment of Helicobacter pylori infection. Helicobacter. 2003;8 Suppl 1:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Anagnostopoulos GK, Kostopoulos P, Margantinis G, Tsiakos S, Arvanitidis D. Omeprazole plus azithromycin and either amoxicillin or tinidazole for eradication of Helicobacter pylori infection. J Clin Gastroenterol. 2003;36:325-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Muthumani K, Zhang D, Dayes NS, Hwang DS, Calarota SA, Choo AY, Boyer JD, Weiner DB. Novel engineered HIV-1 East African Clade-A gp160 plasmid construct induces strong humoral and cell-mediated immune responses in vivo. Virology. 2003;314:134-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Soboll G, Horohov DW, Aldridge BM, Olsen CW, McGregor MW, Drape RJ, Macklin MD, Swain WF, Lunn DP. Regional antibody and cellular immune responses to equine influenza virus infection, and particle mediated DNA vaccination. Vet Immunol Immunopathol. 2003;94:47-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Lodmell DL, Parnell MJ, Bailey JR, Ewalt LC, Hanlon CA. Rabies DNA vaccination of non-human primates: post-exposure studies using gene gun methodology that accelerates induction of neutralizing antibody and enhances neutralizing antibody titers. Vaccine. 2002;20:2221-2228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Carvalho LJ, Daniel-Ribeiro CT, Goto H. Malaria vaccine: candidate antigens, mechanisms, constraints and prospects. Scand J Immunol. 2002;56:327-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Ha SJ, Jeon BY, Kim SC, Kim DJ, Song MK, Sung YC, Cho SN. Therapeutic effect of DNA vaccines combined with chemotherapy in a latent infection model after aerosol infection of mice with Mycobacterium tuberculosis. Gene Ther. 2003;10:1592-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Donnelly J, Berry K, Ulmer JB. Technical and regulatory hurdles for DNA vaccines. Int J Parasitol. 2003;33:457-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Abdelnoor AM. Plasmid DNA vaccines. Curr Drug Targets Immune Endocr Metabol Disord. 2001;1:79-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Henke A. DNA immunization--a new chance in vaccine research? Med Microbiol Immunol. 2002;191:187-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Durrani Z, Rijpkema S. Orogastric vaccination of guinea pigs with Helicobacter pylori sonicate and a high dose of cholera toxin lowers the burden of infection. FEMS Immunol Med Microbiol. 2003;36:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Guy B, Hessler C, Fourage S, Haensler J, Vialon-Lafay E, Rokbi B, Millet MJ. Systemic immunization with urease protects mice against Helicobacter pylori infection. Vaccine. 1998;16:850-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 96] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Keenan JI, Rijpkema SG, Durrani Z, Roake JA. Differences in immunogenicity and protection in mice and guinea pigs following intranasal immunization with Helicobacter pylori outer membrane antigens. FEMS Immunol Med Microbiol. 2003;36:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Dieterich C, Bouzourène H, Blum AL, Corthésy-Theulaz IE. Urease-based mucosal immunization against Helicobacter heilmannii infection induces corpus atrophy in mice. Infect Immun. 1999;67:6206-6209. [PubMed] |
| 20. | Voland P, Hafsi N, Zeitner M, Laforsch S, Wagner H, Prinz C. Antigenic properties of HpaA and Omp18, two outer membrane proteins of Helicobacter pylori. Infect Immun. 2003;71:3837-3843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Lundstrom A, Bolin I, Bystrom M, Nystrom S. Recombinant HpaA purified from Escherichia coli has biological properties similar to those of native Helicobacter pylori HpaA. APMIS. 2003;111:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Lundström AM, Blom K, Sundaeus V, Bölin I. HpaA shows variable surface localization but the gene expression is similar in different Helicobacter pylori strains. Microb Pathog. 2001;31:243-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Evans DG, Queiroz DM, Mendes EN, Svennerholm AM, Evans DJ. Differences among Helicobacter pylori strains isolated from three different populations and demonstrated by restriction enzyme analysis of an internal fragment of the conserved gene hpaA. Helicobacter. 1999;4:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Thole JE, van Dalen PJ, Havenith CE, Pouwels PH, Seegers JF, Tielen FD, van der Zee MD, Zegers ND, Shaw M. Live bacterial delivery systems for development of mucosal vaccines. Curr Opin Mol Ther. 2000;2:94-99. [PubMed] |
| 25. | Dietrich G, Spreng S, Favre D, Viret JF, Guzman CA. Live attenuated bacteria as vectors to deliver plasmid DNA vaccines. Curr Opin Mol Ther. 2003;5:10-19. [PubMed] |
| 26. | Sirard JC, Niedergang F, Kraehenbuhl JP. Live attenuated Salmonella: a paradigm of mucosal vaccines. Immunol Rev. 1999;171:5-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
Edited by Wang XL Proofread by Chen WW