Published online May 1, 2004. doi: 10.3748/wjg.v10.i9.1353
Revised: December 2, 2003
Accepted: December 24, 2003
Published online: May 1, 2004
AIM: To investigate the serum erythropoietin (Epo) levels in patients with chronic liver diseases and to compare to subjects with iron-deficiency anaemia and healthy controls.
METHODS: We examined 31 anaemic (ALC) and 22 non-anaemic (NALC) cirrhotic patients, 21 non- anaemic subjects with chronic active hepatitis (CAH), 24 patients with iron-deficiency anaemia (ID) and 15 healthy controls. Circulating Epo levels (ELISA; R&D Systems, Europe Ltd, Abingdon, UK) and haemoglobin (Hb) concentration were determined in all subjects.
RESULTS: Mean ± SD of Epo values was 26.9±10.8 mU/mL in ALC patients, 12.5 ± 8.0 mU/mL in NALC subjects, 11.6 ± 6.3 mU/mL in CAH patients, 56.4 ± 12.7 mU/mL in the cases of ID and 9.3 ± 2.6 mU/mL in controls. No significant difference (P > 0.05) was found in Epo levels between controls, CAH and NALC patients. ALC individuals had higher Epo levels (P < 0.01) than these groups whereas ID subjects had even higher levels (P < 0.001) than patients suffering from ALC.
CONCLUSION: Increased Epo values in cirrhotics, are only detectable when haemoglobin was lesser than 12 g/dL. Nevertheless, this rise in value is lower than that observed in anaemic patients with iron-deficiency and appears blunted and inadequate in comparison to the degree of anaemia.
- Citation: Bruno CM, Neri S, Sciacca C, Bertino G, Prima PD, Cilio D, Pellicano R, Caruso L, Cristaldi R. Plasma erythropoietin levels in anaemic and non-anaemic patients with chronic liver diseases. World J Gastroenterol 2004; 10(9): 1353-1356
- URL: https://www.wjgnet.com/1007-9327/full/v10/i9/1353.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i9.1353
Chronic anaemia is often observed in patients with liver disease, especially in advanced stages, and an inverse relation has been reported between haemoglobin (Hb) concentration or hematocrit value and survivorship[1]. Inapparent gastrointestinal bleeding, folate and vitamin B12 deficiency, autoimmune haemolysis, altered oxide-reductive balance and hypersplenism are underlying mechanisms responsible for the anaemic state[2-4]. Moreover, a reduced proliferation of erythroid precursor cells has been described in the bone marrow of these patients[4,5]. Erythropoietin (Epo) is an endogenous glycoprotein stimulating erythrocytosis which interacts with erythroid progenitor cells to promote their proliferation and maintain their viability as they differentiate[6,7]. The regulation of erythropoiesis is a biological feedback loop whereby the degree of tissue oxygenation sets the amount of Epo production, the concentration of erythropoietin in turn drives the bone marrow to produce a level of red cells at which oxygen delivery is sufficient to lower Epo production[8]. The gene codifying for this growth factor has been isolated and a regulatory region involved with oxygen sensing has been defined. Expression in response to hypoxia is mediated via a DNA-binding complex[8]. Since literature data[9,10] suggest an emerging role of this hormone in causing haematological changes in chronic diseases, suboptimal production of, or response to, Epo might contribute to the pathogenesis of chronic anaemia in cirrhotics. Some studies regarding the association between Epo levels and cirrhosis have appeared in literature[11-16] but reported data are controversial.
The aim of this study was to investigate the circulating Epo levels in patients suffering from chronic liver disease of various degrees, with and without concomitant anaemia, compared to subjects with iron-deficiency uncomplicated anaemia as well as to healthy controls, in order to assess the relationship between serum Epo and Hb concentration.
We examined 74 patients suffering from chronic liver diseases (21 chronic active hepatitis [CAH] and 53 cirrhosis), 24 patients with iron-deficiency (ID) uncomplicated anaemia and 15 healthy control subjects, comparable to sex and age. Haematology, albumin concentration, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels, serum bilirubin and prothrombin time (as percentage of prothrombin activity), were measured in all patients and controls.
Diagnosis of patients with liver disease, was based on clinical (medical history, physical examination), instrumental (ultrasonography, endoscopy) and laboratory (liver function tests) data. In 47 out of these cases, hepatic damage was confirmed by liver biopsy (in the remaining subjects the procedure was not necessary, as diagnosis was clinically evident).
In 6 out of 21 CAH patients, the etiological agent was hepatitis B virus (HBV) while in the remaining 15, hepatitis C virus (HCV). Of the 53 cirrhotic subjects, 19 were infected with HBV, 31 with HCV while 3 had a history of alcohol abuse.
According to accepted criteria[8], thirty-one cirrhotic patients had normocromic normocytic anaemia (ALC group, mean ± SD Hb 10.2 ± 0.9 gr/dL) and twenty-two were non anemic (NALC group, mean ± SD Hb 13.6 ± 0.7 gr/dL).
None of anaemic cirrhotics had signs of iron-deficiency (serum iron ≥ 70 µg/dL, serum ferritin ≥ 50 ng/mL and transferrin saturation ≥ 30%).
In agreement with Child-Pugh’s classification[17], 12 of NALC patients were in class B and 10 belonged to class C. In the ALC group, 13 patients were in class B whereas 18 in class C.
Cirrhotic subjects affected by gastrointestinal bleeding in the previous 3 mo (as confirmed by endoscopy and fecal occult blood testing) and those with suspected hepatocellular carcinoma (on the basis of ultrasonography, alpha-foetoprotein and carcinoembryonic antigen levels performed during the screening) were excluded from the study.
Diagnosis in patients with iron-deficiency anaemia, was based on clinical (medical history, physical examination), and laboratory (Hb concentration < 12 g/dL, serum ferritin < 40 ng/dL and transferrin saturation < 16%) data. None of them had clinical or laboratory (C-reactive protein) evidence of inflammatory condition.
All subjects had normal renal function (serum creatinine < 1.2 mg/dL, creatinine clearance ≥ 70 mL/min). The main characteristics of our study groups are summarised in Table 1.
| Controls, n = 15 | CAH patients, n = 21 | NALC patients, n = 22 | ALC patiens, n = 31 | ID patients, n = 24 | |
| Age (yr, mean ± SD) | 52 ± 6 | 54 ± 8 | 59 ± 3 | 60 ± 5 | 54 ± 5 |
| Males | 8 | 10 | 9 | 12 | 10 |
| Famales | 7 | 11 | 13 | 19 | 14 |
| Albumin (g/dL) | 3.6 ± 0.2 | 3.3 ± 0.3 | 3.0 ± 0.3 | 2.9 ± 0.2 | 3.5 ± 0.3 |
| AST (U/L) | 14.2 ± 1.5 | 73.7 ± 19.2 | 41.9 ± 12.6 | 40.6 ± 16.4 | 16.1 ± 3.5 |
| ALT (U/L) | 15.6 ± 2.7 | 69.7 ± 8.4 | 39.8 ± 10.1 | 37.9 ± 12.5 | 14.2 ± 3.1 |
| Bilirubin (mg/dL) | 0.6 ± 0.1 | 0.8 ± 0.1 | 1.1 ± 0.2 | 1.3 ± 0.2 | 0.7 ± 0.1 |
| Prothrombin time (%) | 98.2 ± 5.0 | 81.1 ± 6.2 | 72.4 ± 8.9 | 70.3 ± 10.2 | 95.1 ± 3.1 |
The study was conformed to Helsinki Declaration and informed consent was obtained from the whole study series.
A blood sample was obtained from all subjects and ethylenediaminetetraacetic acid (EDTA) was added. Samples were centrifuged and plasma was stored at -25 °C. Erythropoietin was determined by a commercial ELISA kit (R&D Systems Europe Ltd, Abingdon, United Kingdom). The sensitivity of assay was less than 0.6 mU/mL. Non specific binding was < 1%. Intra and inter-assay variability averaged 3.1% and 3.5%, respectively. Epo concentrations were expressed as mU/mL.
Analysis of variance and Kruskall-Wallis test were used to compare mean±SD between various groups. Relationship between continuous variables was investigated by correlation test or multiple regression test when they were more than two. Covariance analysis was performed to assess the difference in Epo values for adjusted Hb, between ALC and ID patients. The regression lines between Hb concentration and Epo values in these two groups were compared by t-test, according to current statistical procedures[18].
No significant difference (P > 0.05) in Hb concentration was observed between controls (13.9 ± 0.3 gr/dL), CAH (13.7 ± 0.5 gr/dL) and NALC (13.6 ± 0.7 gr/dL) patients. Subjects suffering from ALC (10.2 ± 0.9 gr/dL) and ID (10.1 ± 0.8 gr/dL) had values significantly lower than others (P < 0.05), but Hb concentration was similar in these two groups (P > 0.05). Among cirrhotic subjects, Hb concentration was similar (P > 0.05) in Child-Pough class B and class C patients both in ALC (10.4 ± 0.7 gr/dL versus 10.1 ± 0.9 gr/dL, respectively) and in NALC (13.8 ± 0.4 gr/dL versus 13.5 ± 0.9 gr/dL, respectively) groups.
Mean ± SD of plasma Epo was 9.3±2.6 mU/mL in controls, 11.6 ± 6.3 mU/mL in CAH patients, 12.5 ± 8.0 mU/mL in NALC subjects, 26.9 ± 10.8 mU/mL in ALC patients and 56.4 ± 12.7 mU/mL in ID individuals.
Regarding Child-Pugh related allocation, mean ± SD of plasma Epo was 24.18 ± 9.56 mU/mL for patients in class B vs 30.71 ± 10.82 mU/mL for those in class C of the ALC group, 12.3 ± 7.8 mU/mL in class B vs 12.9 ± 8.1 mU/mL in class C patients of the NALC group, respectively.
Statistical analysis did not show any significant difference between controls, CAH patients and NALC patients (P > 0.05). ALC subjects had significantly higher Epo levels than these three groups (P < 0.01) and patients suffering from ID had significantly higher Epo levels than ALC patients (P < 0.001). In both NALC and ALC groups, no significant difference (P > 0.05) was observed between class B and class C patients.
Epo levels were not related (P > 0.05) to albumin concentration, AST and ALT values, serum bilirubin and prothrombine time in any group of patients with liver disease.
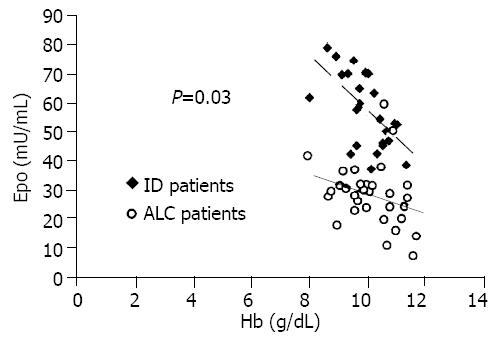
An inverse significant relationship between Epo and Hb was found in ID patients (r = -0.61, P = 0.001) but not in ALC patients (r = -0.22, P > 0.05). Covariance analysis revealed a significant difference (P < 0.001) in Epo values for adjusted Hb concentration between ALC and ID patients.
Finally, the regression lines between Epo and Hb in ALC and ID patients, were compared and a significant difference was found (ALC patients: slope -2.47, ES 2.02, y-intercept 52.39; ID patients: slope -9.79, ES 2.7, y-intercept 155.48; t = 2.23, fd = 53, P = 0.03).
Anaemia is a multifactorial complication of liver cirrhosis[2-5]. Papers regarding abnormalities of circulating Epo in patients with liver diseases were few and results were contradictory[11-18]. Some authors have reported higher Epo levels in cirrhotic patients when compared to healthy controls. An inverse relation between Epo values and the haematological indices has been described by some groups.
We assessed plasma Epo levels in anaemic and non-anaemic patients with chronic liver disease compared to healthy controls and iron-deficiency anaemic subjects.
Our results showed that non-anaemic patients with liver disease and controls had similar Epo values, in cirrhotic patients, circulating Epo was not related to Child-Pugh score. Moreover, multiple regression test documented that Epo levels were not related to concentration of albumin, bilirubin, ALT or AST values and prothrombin time in any of these three groups of patients with liver disease.
This was in part conflicting with the findings of other authors[12,13,15] and suggested that liver damage itself, independent of the degree of dysfunction, was not able to alter circulating Epo levels.
On the other hand, we detected a significant increase in levels of this hormone both in ALC patients and in ID patients. However, a significant inverse correlation between Epo and Hb was found in ID group (r = -0.61, P < 0.05) but not in ALC group (r = -0.22, P > 0.05).
Nevertheless, increased Epo level in anaemic patients versus healthy controls was not enough to assess Epo production.
In fact, the definition of defective Epo production has relied on a low Epo value in comparison to reference patients with similar Hb[19]. Consequently, circulating Epo cannot be simply compared with normal values and levels found must be evaluated in relation to the degree of anaemia.
As it has been widely accepted that adequate Epo production occur in patients with iron-deficiency anaemia[19], we chosen ID patients as reference group.
Mean±SD of Hb values was similar in the two groups of anaemic patients, but mean ± SD of Epo concentrations in ALC group was much lower than that in ID patients.
Analysis of covariance showned a significant difference in Epo values between the two groups (P < 0.001). Moreover, we also compared the regression lines between Epo and Hb in ALC and ID patients. The slope and y-intercept of two regression lines (Figure 1) were significantly different (P < 0.05).
Therefore, although circulating Epo levels in ALC patients, were higher than that in non anaemic individuals with liver disease, they were significantly lower in healthy subjects, than in ID patients. The reason of this finding is unclear. It seems that an altered Epo clearance did not result in the difference because normal metabolism of this hormone was maintained in cirrhotics[20].
Cazzola et al[21] described an inverse relationship between red blood precursor mass and serum Epo. As in cirrhosis, a reduction of erythroid cells has been reported[4,5], the finding of lower Epo values in our ALC patients than in ID patients was not due to an increased utilization by precursor cells.
Therefore, lower Epo values in ALC subjects, are likely provoked by an impaired synthesis rather than by an increased metabolism.
Consequently, we think that Epo production occurred in different quantitative patterns in these two groups, and other factors besides Hb concentration, could affect its output in cirrhotic patients.
This can explain why no relationship was found between Hb and Epo in our ALC patients.
In adults, Epo has been found to be synthesised by the kidney and to a lesser extent by the liver[22]. Thus, liver failure could endanger the hepatic residual share of Epo synthesis, even though in this study, the absence of relation between Epo values and investigated indices of liver dysfunction did not support this hypothesis. Alternatively, a reduced sensitivity of renal cells to hypoxic stimuli could be hypothesized. Furthermore, the inhibitory action of reactive oxygen species and nitric oxide as well as malnutrition of cirrhotic patients might contribute to reduction in Epo synthesis[23-26].
Finally, inflammatory cytokines, namely interleukin-1, tumor necrosis factor and transforming growth factor, are enhanced in liver diseases and have been found to inhibit hypoxia-induced erythropoietin production in vitro and in vivo[27-29].
Whatever the cause of blunted and unsuitable rise in circulating Epo is the levels of this hormone in anaemic cirrhotics, appear defective and inadequate with regard to the degree of anaemia.
Even though Epo deficiency is not only the cause of anaemia in cirrhotics, its insufficient concentration could play a role in the persistence of anaemic status, worsening the outcome of cirrhotic patients.
In conclusion, non-anaemic patients with chronic liver disease have normal Epo levels. During cirrhosis, elevated Epo values are detectable in patients with Hb concentration lesser than 12 g/dL and are not related to the degree of both Hb concentration and severity of liver dysfunction. In addition, such an increase is lower than that observed in patients with iron-deficiency anaemia and appears blunted and inadequate in comparison to the degree of anaemia.
| 1. | Pignon JP, Poynard T, Naveau S, Marteau P, Zourabichvili O, Chaput JC. [Multidimensional analysis by Cox's model of the survival of patients with alcoholic cirrhosis]. Gastroenterol Clin Biol. 1986;10:461-467. |
| 2. | Tymofieiev VV, Kolomoiets' MIu. [The pathogenetic characteristics of the anemic syndrome in liver cirrhosis]. Lik Sprava. 1997;66-71. |
| 3. | Mehta AB, McIntyre N. Haematological disorders in liver disease. Forum (Genova). 1998;8:8-25. |
| 4. | Sherlok S, Dooley J. "The haematology of liver disease" in Diseases of the Liver and Biliary System. 11th ed. Oxford: Blackwell Publishing 2002; 47. |
| 5. | Korolko IuR, Sarycheva TG, Kotelńikov VM, Kozinets GI, Zherebtsov LA. [Anemic syndrome in chronic hepatitis and liver cirrhosis]. Klin Med (Mosk). 1993;71:45-48. |
| 6. | Spivak JL, Pham T, Isaacs M, Hankins WD. Erythropoietin is both a mitogen and a survival factor. Blood. 1991;77:1228-1233. |
| 7. | Koury MJ, Bondurant MC. Erythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cells. Science. 1990;248:378-381. |
| 9. | Scudla V, Adam Z, Scudlová M. [Diagnosis and therapy of anemia in chronic diseases]. Vnitr Lek. 2001;47:400-406. |
| 11. | Siciliano M, Tomasello D, Milani A, Ricerca BM, Storti S, Rossi L. Reduced serum levels of immunoreactive erythropoietin in patients with cirrhosis and chronic anemia. Hepatology. 1995;22:1132-1135. |
| 12. | Pirisi M, Fabris C, Falleti E, Soardo G, Toniutto P, Gonano F, Bartoli E. Evidence for a multifactorial control of serum erythropoietin concentration in liver disease. Clin Chim Acta. 1993;219:47-55. |
| 13. | Mady E, Wissa G, Khalifa A, el-Sabbagh M. Assessment of erythropoietin levels and some iron indices in chronic renal failure and liver cirrhosis patients. Dis Markers. 1999;15:229-236. |
| 14. | Vasilopoulos S, Hally R, Caro J, Martin P, Westerberg S, Moritz M, Jarrell B, Muñoz S. Erythropoietin response to post-liver transplantation anemia. Liver Transpl. 2000;6:349-355. |
| 15. | Tacke F, Schoffski P, Gauser A, Manus MP. Erythropoietin plasma levels are elevated in chronic liver disease and correlate with anemia, liver dysfunction, bleeding episodes and interleukin-6 (Abstract). J Hepatol. 2002;36:65. |
| 16. | Oczko-Grzesik B, Wiecek A, Kokot F. Influence of IFN-alpha on plasma erythropoietin levels in patients with hepatitis B virus-associated chronic active hepatitis. J Interferon Cytokine Res. 2001;21:669-676. |
| 17. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. |
| 18. | Colton T. Statistics in Medicine. Boston: Little, Brown and Company 1973; 201. |
| 19. | Cazzola M, Mercuriali F, Brugnara C. Use of recombinant human erythropoietin outside the setting of uremia. Blood. 1997;89:4248-4267. |
| 20. | Jensen JD, Jensen LW, Madsen JK, Poulsen L. The metabolism of erythropoietin in liver cirrhosis patients compared with healthy volunteers. Eur J Haematol. 1995;54:111-116. |
| 21. | Cazzola M, Guarnone R, Cerani P, Centenara E, Rovati A, Beguin Y. Red blood cell precursor mass as an independent determinant of serum erythropoietin level. Blood. 1998;91:2139-2145. |
| 23. | Canbolat O, Fandrey J, Jelkmann W. Effects of modulators of the production and degradation of hydrogen peroxide on erythropoietin synthesis. Respir Physiol. 1998;114:175-183. |
| 24. | Fandrey J, Frede S, Jelkmann W. Role of hydrogen peroxide in hypoxia-induced erythropoietin production. Biochem J. 1994;303:507-510. |
| 25. | Schobersberger W, Hoffmann G, Fandrey J. Nitric oxide donors suppress erythropoietin production in vitro. Pflugers Arch. 1996;432:980-985. |
| 26. | Genius J, Fandrey J. Nitric oxide affects the production of reactive oxygen species in hepatoma cells: implications for the process of oxygen sensing. Free Radic Biol Med. 2000;29:515-521. |
| 27. | Faquin WC, Schneider TJ, Goldberg MA. Effect of inflammatory cytokines on hypoxia-induced erythropoietin production. Blood. 1992;79:1987-1994. |
| 28. | Jelkmann WE, Fandrey J, Frede S, Pagel H. Inhibition of erythropoietin production by cytokines. Implications for the anemia involved in inflammatory states. Ann N Y Acad Sci. 1994;718:300-309; discussion 309-311. |
| 29. | Poveda Gómez F, Camacho Siles J, Quevedo Morales E, Fernández Zamorano A, Codoceo Alquinta R, Arnalich Fernández F, Sempere Alcocer M. [Pattern of blood levels of erythropoietin and proinflammatory cytokines in patients with anemia of chronic disorders secondary to infection]. An Med Interna. 2001;18:298-304. |
Edited by Wang XL, Xu FM