Published online May 1, 2004. doi: 10.3748/wjg.v10.i9.1321
Revised: November 2, 2003
Accepted: December 24, 2003
Published online: May 1, 2004
AIM: To study the blood-brain barrier integrity, brain edema, animal behavior and ammonia plasma levels in prehepatic portal hypertensive rats with and without acute liver intoxication.
METHODS: Adults male Wistar rats were divided into four groups. Group I: sham operation; II: Prehepatic portal hypertension, produced by partial portal vein ligation; III: Acetaminophen intoxication and IV: Prehepatic portal hypertension plus acetaminophen. Acetaminophen was administered to produce acute hepatic injury. Portal pressure, liver serum enzymes and ammonia plasma levels were determined. Brain cortex water content was registered and trypan blue was utilized to study blood brain barrier integrity. Reflexes and behavioral tests were recorded.
RESULTS: Portal hypertension was significantly elevated in groups II and IV. Liver enzymes and ammonia plasma levels were increased in groups II, III and IV. Prehepatic portal hypertension (group II), acetaminophen intoxication (group III) and both (group IV) had changes in the blood brain-barrier integrity (trypan blue) and hyperammonemia. Cortical edema was present in rats with acute hepatic injury in groups III and IV. Behavioral test (rota rod) was altered in group IV.
CONCLUSION: These results suggest the possibility of another pathway for cortical edema production because blood brain barrier was altered (vasogenic) and hyperammonemia was registered (cytotoxic). Group IV, with behavioral altered test, can be considered as a model for study at an early stage of portal-systemic encephalopathy.
- Citation: Scorticati C, Prestifilippo JP, Eizayaga FX, Castro JL, Romay S, Fernández MA, Lemberg A, Perazzo JC. Hyperammonemia, brain edema and blood-brain barrier alterations in prehepatic portal hypertensive rats and paracetamol intoxication. World J Gastroenterol 2004; 10(9): 1321-1324
- URL: https://www.wjgnet.com/1007-9327/full/v10/i9/1321.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i9.1321
Portal hypertension (PH) is a major syndrome that frequently accompanies chronic liver diseases such as cirrhosis. Prehepatic PH creates a splanchnic hyperdynamic circulation and hyperemia with increased splanchnic resistance and production of collateral vessels that drive splanchnic blood flow to systemic circulation[1].
Portal-systemic encephalopathy (PSE), the most commonly encountered form of hepatic encephalopathy (HE), describes a wide spectrum of reversible and irreversible neuropsychiatric abnormalities that appear as a complication in patients with acute or chronic liver disease. HE is usually found as an overt form or in a mild form with lesser neuropsychiatric symptoms, which can be misdiagnosed[2].
Acetaminophen (APAP) is a non-steroid anti-inflammatory drug (NSAID), frequently used in adults and children. At high concentration of APAP, the gluthation-dependent conjugation system generates a toxic metabolite that binds covalently to cellular proteins and macromolecules followed by cell destruction[3,4].
Finally, ammonia originated from the gut protein metabolism has been implicated as an important factor in the production of HE. In chronic liver disease, ammonia evades liver catabolism to urea through portal systemic shunts and collaterals, and reaches the brain in high blood concentration[5].
In previous experiments we documented different aspects of the relationship between prehepatic PH in rats and central nervous system: alterations in uptake and release of norepinephrine and modification and tyrosine hydroxylase activity in discrete regional mesencephalic nucleus[6,7]. Furthermore, we considered that PH rats underwent a subclinical HE[8].
The aim of this experiment was to study the blood-brain barrier (BBB) integrity, brain cortical edema, ammonia plasma levels and behavior in rats with different liver injuries.
Male Wistar rats (240-260 g of body mass, 12 h of light cycle: 8 a.m.-8 p.m.) were used and animal welfare was in accordance with guidelines of the Faculty of Pharmacy and Biochemistry of Buenos Aires and approved by ethical committee according to Helsinki’s Declaration. Animals were placed in individual cages and allowed to recover from surgery. Rats were fed standard laboratory chow and water ad libitum.
Group II (chronic PH): Portal hypertensive (PH) animals were obtained by calibrated stenosis of the portal vein according to Chojkier et al[1]. Rats were lightly anesthetized with ether and then midline abdominal incision was made. The portal vein was located and isolated from surrounding tissues. A ligature of 3.0 silk suture was placed around the vein, and snuggly tied to a 20-gauge blunt-end needle placed along the side of the portal vein. The needle was subsequently removed to yield a calibrated stenosis of portal vein, after which the abdomen was sutured. Operations were performed at 2 p.m. to obey circadian rhythm and fourteen days later the animals developed PH.
Group III (acute intoxication): Acetaminophen was injected i.p. at a dose of 750 mg/kg.d per rat on the 13th and 14th day, considering as the start day or day zero, the day of the surgical procedure of group II.
Group IV (chronic PH plus acute intoxication): Rats underwent partial portal vein ligation as in group II and then received acetaminophen the same dose as in group III.
Group I: Shamly operated rats underwent the same experimental procedure like group II, except that portal vein was isolated but not stenosed.
All groups received a subcutaneous injection (25 mL/kg of body mass) of a 50 g/L dextrose, 4.5 g/L saline solution, containing 20 mEq/L of potassium chloride, every 12 h after the first injection of APAP, as a supportive therapy to prevent hypoglycemia and renal failure[9]. Rats were sacrificed on d 14, 6 to 8 h after the last acetaminophen injection.
Following experiment were performed in four groups of animals (I: SHAM, II: HP, III: APAP and IV: HP + APAP, n = 6/8 per group).
Rats were anesthetized with sodium pentobarbital (40 mg/kg, ip). The spleen was cannulated with a polyethylene cannula (PE 50) filled with heparinized saline solution (25 U/mL) to measure portal pressure by Statham Gould P23ID pressure transducer (Statham, Hato Rey, Puerto Rico) coupled to a Grass 79 D polygraph (Grass Instruments, Quincy, MA).
Blood samples were obtained by abdominal aortic artery puncture for the determination of biochemical parameters. Plasma aminotransferase activity was determined using standardized and optimized commercial Boheringer-Manenhein kits (Germany). Ammoniac Enzyimatique UV kits (Biomerieux-France) were used to determine plasma ammonia concentration. Plasma levels of urea, glucose and creatinine were measured by current biochemical colorimetric UV methods (Wiener, Argentine).
High resolution optical microscopy (HROM): sections of liver were fixed in buffered formalin and embedded in paraplast. Routine stains were used: hematoxylin-eosin, PAS and Masson’s trichrome for light microscopy and toluidine blue stain for HROM.
Rats were sacrificed by exsanguination under complete ether anesthesia, their brains cortex water content was measured to quantify possible brain edema according to Marmorou[10].
One cerebral hemisphere was quickly removed after rats were sacrificed and stored at 4 °C to be processed within 30 min. The hemisphere was cut into coronal slices and eight samples taken from the cerebral cortex, approximately 10 mg in weight, was placed in a bromobenzene-kerosene density gradient column to measure brain water[10]. The column was previously calibrated with varying concentrations of potassium sulfate to ensure a linear relation (> 0.997) between the K2 SO4 and readings in the density gradient. The equilibration point of the samples was read at 2 min and averaged, conversion from specific gravity to brain water was done as previously reported[10].
Rats were perfused with trypan blue (TB) solution and then fixed in paraformaldehyde. TB solution (5 g/L) was made by dissolving 1 g of TB in 200 mL of PBS with gentle heat. The solution was allowed to cool room temperature and then added to the filtrate, then the solution was placed on ice and used immediately. The temperature of TB solution was 10 °C-12 °C at the time of perfusion. Rats were anesthetized with ethyl urethane (1 mg/kg) and perfused transcardially with 200 mL of TB solution, followed by 300 mL of ice-cold paraformaldehyde (20 g/L in PBS), the low rate of perfusate was maintained at 25 mL/min. Brains were dissected and post-fixed overnight in 0.3 kg/L sucrose for 2 d. Subsequently, brains were frozen in powdered dry ice and stored at -80 °C until processed for microscopic studies. Slices of brain hippocampus were obtained with cryostat in sections of 300 microns according to Watson and Paxinos (Hippocampus fig 24, bregma 4.8, interneuron 4.2). Hippocampal slices were evaluated under light microscopy and the results were expressed as positive (+) or negative (-) for TB staining. Medial eminence and choroids plexus staining were used as control of TB appropriated perfusion[11].
Corneal, pain-response and right reflexes were performed. Automated open field (Animex) and rota-rod tests (to study motor coordination) were realized. Rota-rod speed was fixed at 25 turns/min. Rats were trained 48 h before the experiment until they fell down less than 3 times in 5 min. The number of falls during the experiment and time elapsed to the first fall were recorded[12,13].
Results were expressed as mean ± SE. Statistical analysis was performed by means of repeated measurement analysis of variance (ANOVA) followed by Newman-Keuls’s test. Dunn’s test was used for non-parametric data, P values less than 0.05 were considered statistically significant.
Portal pressure (PP) was significant higher in groups II and IV (aP < 0.01) when compared to control group. There was no significant difference when compared PP of group I (Sham) with group III (APAP) (Table 1).
AST plasma levels were significant increased in groups II, III and IV when compared with group I (bP < 0.001). ALT plasma levels were significant increased in groups III and IV when compared with control group (Table 1).
Plasma levels of urea, glucose and creatinine were normal in all groups of rats (data not shown).
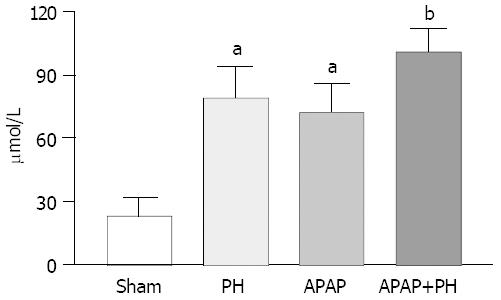
Ammonia blood concentrations (μmol/L) showed significant increase in PH rats, APAP group and PH plus APAP (P < 0.05 and P < 0.001 respectively) (Figure 1).
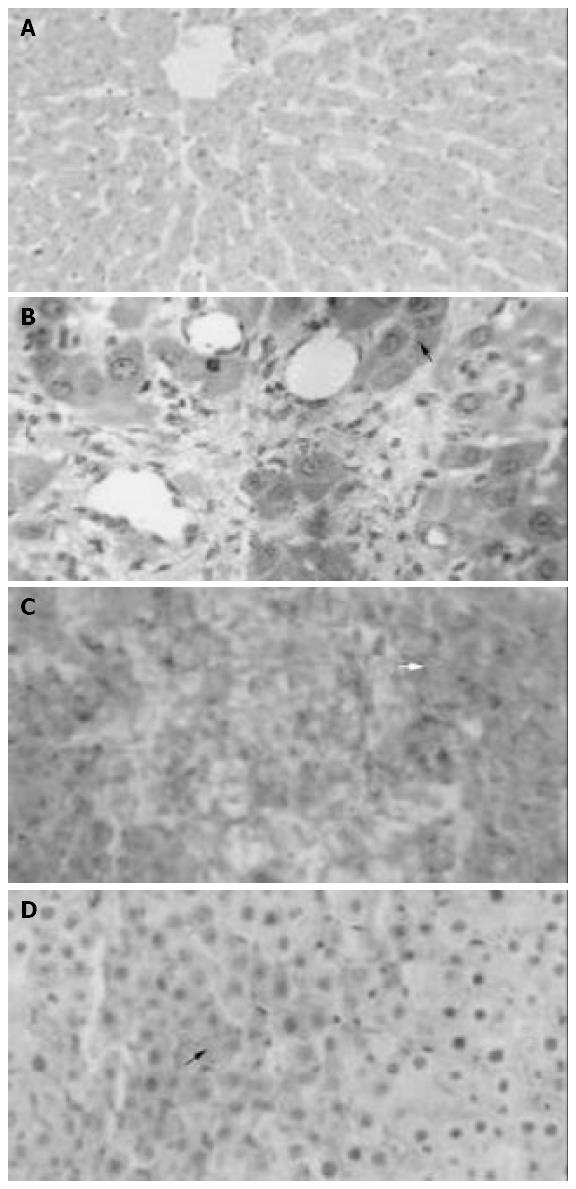
Light microscopy and HROM showed normal histology in liver parenchyma in group I, minimal focal necrosis in group II; diffuse hemorrhagic necrosis in group III and focal hemorrhage and confluent necrosis in group IV (Figure 2A-D).
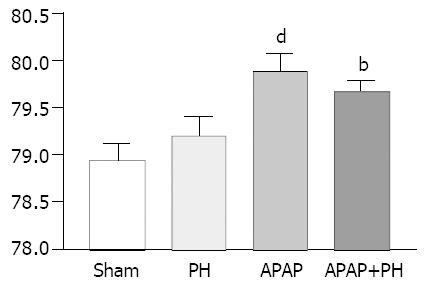
Brain cortical water content (H2O/g brain weight %) showed significant increase in rats injected with APAP (P < 0.001) and in those with PH + APAP (P < 0.01) (Figure 3).
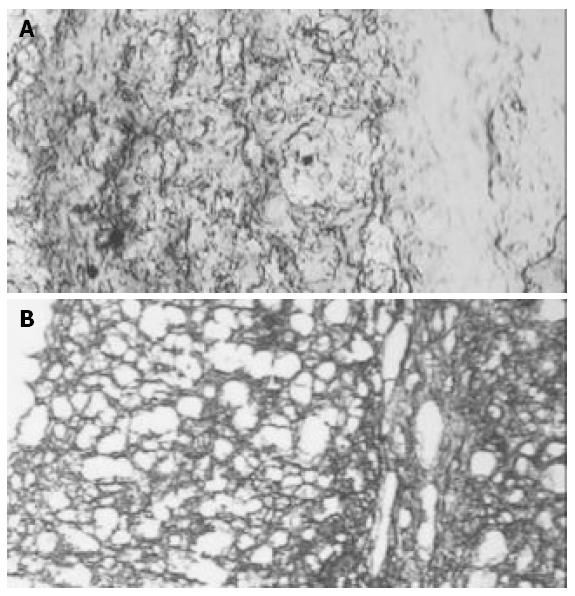
In Groups II, III and IV trypan blue was positive in the perivascular space in the hippocampus area (Figure 4).
All reflexes studied including the Animex were normal for all groups except for rota-rod test that showed in group IV a significant difference at the time elapsed to the first fall (not in number of falls), when compared to shamly operated animals (Table 2).
Experimental prehepatic portal hypertension produces a hyperdynamic redistribution of splanchnic circulation and minimal liver damage. It could be considered that prehepatic PH is a subclinic PSE stage[8]. In this study APAP was used to produce liver injury because of its aggressive effect on liver parenchyma[3,4]. It was administrated to two groups that reflected different situations, namely an acute form of liver injury (group III) and a chronic liver PH group plus an acute liver intoxication.
Portal pressure (PP) was significantly increased in group II, as it was described elsewhere[8] and in group IV. PP in group III (APAP) had normal values. This agreed with acute intoxications and specifically with acute APAP intoxication[14]. Group IV and group II had similar PP values, and it might be due to a chronic PH induced shunt (groups II and IV).
Liver enzymes reflected hepatocyte damage. When liver parenchyma was injured, the enzymes increased as it could be seen in early stages of PSE. Plasma enzyme levels were higher in group III than those in APAP induced acute liver damage. In addition, liver histopathology was concordant with these data as a diffuse hemorrhagic necrosis was observed in group III[14].
Ammonia plasma level data showed normal values in group I and significantly increased values in groups II, III and IV. The values registered were remarkably lower than those observed in different models of acute liver failure (ALF). It is important to point out that in group II hyperammonemia was produced by opened shunts due to the splanchnic blood flow redistribution[15]. It is also interesting that in this experimental model the hepatic blood flow was reduced, but not completely shunted as in other models (e.g., porto-caval shunts, PCS). Ammonia was markedly increased as the major responsible molecule related to HE[16] had several pathways, lately the cGMP-NO-glutamate that leads to cytotoxic brain edema characterized by swelling of astrocytes and their processes, raised intracranial pressure and as a consequence, to brain herniation[5]. This was the main cause of mortality in ALF and it could be predicted by increased arterial ammonia plasma levels[17]. Besides this, PSE could lead to increased nNOS activity[18], associated with L-arginine uptake in a PCS model[19].
In this model of PH, brain edema was not correlated with ammonia increase. Furthermore brain edema was documented in group III and ammonia level was lower than that in group II and no cerebral edema was observed. Besides this, brain edema was lower in group IV than in group III and the highest recorded ammonia was recorded. What is the major mechanism involved in the development of brain edema in this experiment? One of the pathways of brain edema is the vasogenic and if that mechanism was involved in groups III and IV, why in group II BBB integrity was broken-down and ammonia plasma levels were increased, and no cortical brain edema was documented? Although a mixed mechanism (vasogenic and cytotoxic) was proposed for brain edema in ALF[20], in this experiment no brain edema was present in the chronic group without acute hepatocyte injury. In the chronic group plus acute liver injury brain edema was registered and the cytotoxic mechanism could be mostly responsible. Under these experimental conditions another mechanism of brain edema might involve aquaporins. The aquaporin-type water channels are integral membrane proteins that have been found to function as conduits for osmotically driven transport of water across cell plasma membranes[21]. In this experiment BBB integrity was altered and thus endothelial cells were involved. AQP4 presented in both, endothelia and astrocytes might play an active role in regulation of astrocyte swelling and the presence of brain edema[22].
TB demonstrated altered integrity of the BBB at the hippocampus areas involved in learning and memory consolidation in groups II, III and IV. Furthermore, the only group with an altered behavioral test, at first fall in rota rod test, was group IV. These might be related with the observation in the only group with PH, elevated enzymes, hepatocyte injury, hyperammonemia, brain edema, positive TB and altered rota rod test. Dixit[23] demonstrated that in rats with galactosamine-induced ALF BBB breakdown evidenced by TB dye infusion studies began in grade III coma. Despite this, under the present experimental conditions no clinical evidence of coma could be registered in any group. Therefore, group IV can be considered as an experimental model of subclinic PSE.
| 1. | Chojkier M, Groszmann RJ. Measurement of portal-systemic shunting in the rat by using gamma-labeled microspheres. Am J Physiol. 1981;240:G371-G375. |
| 2. | Watanabe A. Cerebral changes in hepatic encephalopathy. J Gastroenterol Hepatol. 1998;13:752-760. |
| 3. | Kelly JH, Koussayer T, He DE, Chong MG, Shang TA, Whisennand HH, Sussman NL. An improved model of acetaminophen-induced fulminant hepatic failure in dogs. Hepatology. 1992;15:329-335. |
| 4. | Francavilla A, Makowka L, Polimeno L, Barone M, Demetris J, Prelich J, Van Thiel DH, Starzl TE. A dog model for acetaminophen-induced fulminant hepatic failure. Gastroenterology. 1989;96:470-478. |
| 5. | Felipo V, Butterworth RF. Neurobiology of ammonia. Prog Neurobiol. 2002;67:259-279. |
| 6. | Lemberg A, Eizayaga FX, Vatta M, Dominguez A, Romay S, Bianciotti LG, Sansó G, Fernández B. Prehepatic portal hypertension in rats modifies norepinephrine metabolism in hypothalamus, medulla oblongata and portal vein. Dig Dis Sci. 1993;38:1259-1262. |
| 7. | Lemberg A, Rubio M, Bengochea L, Romay S, Eizayaga F, Diez A, Perazzo JC. Tyrosine hydroxilase activity in discrete brain regions from prehepatic portal hypertensive rats. Hepatogastroenterology. 1998;45:547-550. |
| 8. | Scorticati C, Prestifilippo JP, Murer G, Lemberg A, Perazzo JC. [Functional alterations in central nervous system of prehepatic portal hypertensive rats]. Medicina (B Aires). 2001;61:673-675. |
| 9. | Gammal SH, Basile AS, Geller D, Skolnick P, Jones EA. Reversal of the behavioral and electrophysiological abnormalities of an animal model of hepatic encephalopathy by benzodiazepine receptor ligands. Hepatology. 1990;11:371-378. |
| 10. | Marmarou A, Poll W, Shulman K, Bhagavan H. A simple gravimetric technique for measurement of cerebral edema. J Neurosurg. 1978;49:530-537. |
| 11. | Reynolds DS, Morton AJ. Changes in blood-brain barrier permeability following neurotoxic lesions of rat brain can be visualised with trypan blue. J Neurosci Methods. 1998;79:115-121. |
| 12. | Dunham NW, Miya TS. A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharm Assoc Am Pharm Assoc. 1957;46:208-209. |
| 13. | Maynert EW. A test group for central depressants. Screening methods in pharmacology. New York: Academic Press 1965; 80-86. |
| 14. | Bengochea L, Ghanem C, Perazzo JC, Ghisolfi C, Marabotto L, Acevedo C, Mino J, Lemberg A, Rubio M. Drug glucuronidation and hepatic lipid microsomal membrane profile in cholestatic rats followed paracetamol intoxication. Pharmacol Res. 1999;40:369-376. |
| 15. | MacMathuna P, Vlavianos P, Westaby D, Williams R. Pathophysiology of portal hypertension. Dig Dis. 1992;10 Suppl 1:3-15. |
| 16. | Kramer L, Tribl B, Gendo A, Zauner C, Schneider B, Ferenci P, Madl C. Partial pressure of ammonia versus ammonia in hepatic encephalopathy. Hepatology. 2000;31:30-34. |
| 17. | Clemmesen JO, Larsen FS, Kondrup J, Hansen BA, Ott P. Cerebral herniation in patients with acute liver failure is correlated with arterial ammonia concentration. Hepatology. 1999;29:648-653. |
| 18. | Rao VL, Audet RM, Butterworth RF. Increased nitric oxide synthase activities and L-[3H]arginine uptake in brain following portacaval anastomosis. J Neurochem. 1995;65:677-678. |
| 19. | Rao VL, Audet RM, Butterworth RF. Portacaval shunting and hyperammonemia stimulate the uptake of L-[3H] arginine but not of L-[3H]nitroarginine into rat brain synaptosomes. J Neurochem. 1997;68:337-343. |
| 20. | Swain M, Butterworth RF, Blei AT. Ammonia and related amino acids in the pathogenesis of brain edema in acute ischemic liver failure in rats. Hepatology. 1992;15:449-453. |
| 21. | Verkman AS. Role of aquaporin water channels in eye function. Exp Eye Res. 2003;76:137-143. |
| 22. | Papadopoulos MC, Krishna S, Verkman AS. Aquaporin water channels and brain edema. Mt Sinai J Med. 2002;69:242-248. |
| 23. | Dixit V, Chang TM. Brain edema and the blood brain barrier in galactosamine-induced fulminant hepatic failure rats. An animal model for evaluation of liver support systems. ASAIO Trans. 1990;36:21-27. |
Edited by Wang XL Proofread by Xu FM