Published online Apr 15, 2004. doi: 10.3748/wjg.v10.i8.1208
Revised: October 1, 2003
Accepted: October 12, 2003
Published online: April 15, 2004
AIM: To investigate whether hepatic progenitor cells (HPC), that reveal the features of oval cells in rodents and small epithelial cells (SEC) in certain human liver disease, were also found in human liver cirrhosis (HLC).
METHODS: Surgical liver specimens from 20 cases of hepatitis B virus-positive HLC (15 cases containing hepatocellular carcinoma) were investigated by light microscopic immunohistochemistry (LM-IHC). Among them specimens from 15 cases were investigated by electron microscopy (EM) and those from 5 cases by immunofluorencence confocal laser scanning microscopy (ICLSM). Antibodies against cytokeratin 7 and albumin were used and single and/or double labelling were performed respectively.
RESULTS: LM-IHC showed that at the margins of regenerating nodules and in the fibrous septae, a small number of cells in the proliferating bile ductules were positive for CK7 and albumin. At the EM level these HPC were morphologically similar to the SEC described previously, and also similar to the oval cells seen in experimental hepatocarcinogenesis. They were characterized by their small size, oval shape, a high nucleus/cytoplasm ratio, a low organelle content in cytoplasm, and existence of tonofilaments and intercellular junctions. ICLSM revealed that HPC expressed both cytokeratin 7 and albumin.
CONCLUSION: HPC with ultrastructural and immunophenotypical features of oval cells, i.e., hepatic stem cell-like cells as noted in other liver diseases, were found in HLC. These findings further support the hypothesis that bipotent hepatic stem cells, that may give rise to biliary epithelial cells and hepatocytes, exist in human livers.
- Citation: Xiao JC, Jin XL, Ruck P, Adam A, Kaiserling E. Hepatic progenitor cells in human liver cirrhosis: Immunohistochemical, electron microscopic and immunofluorencence confocal microscopic findings. World J Gastroenterol 2004; 10(8): 1208-1211
- URL: https://www.wjgnet.com/1007-9327/full/v10/i8/1208.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i8.1208
In rodents, administration of hepatocarcinogens could lead to proliferation of oval cells that are capable of differentiating towards both hepatocytes and bile duct epithelium. These oval cells are thought to be hepatic progenitor cells or stem cells[1-4]. In contrast to the situation in rodents, it is still controversial, however, whether hepatic progenitor cells also exist in human livers. Recently, we described a population of cells with the morphological and immunophenotypical features of oval cells in hepatoblastoma (HB) that we termed small epithelial cells (SEC)[5-6]. In further studies we also observed similar cells in livers of patients with hepatocellular carcinoma (HCC)[7], extrahepatic biliary atresia (EBA)[8] and HLC[9].
SEC reveal certain morphological features, such as a small size, a high nucleus to cytoplasm ratio, intercellular junctions and tonofilaments. Under immunoelectron microscopy, SEC were found to express both cytokeratin 7 (a marker of biliary differentiation), and albumin (a marker of hepatocytic differentiation)[5-9]. Thus SEC exhibit morphological and immunophenotypical features of oval cells in rodents. This study was undertaken to further verify that SEC did occur in hepatitis B virus (HBV)-positive HLC and that such cells played a key role in the pathogenesis of the disease. The findings further support the hypothesis of the existence of human hepatic stem cells.
Fifteen surgical specimens of cirrhotic liver tissue adjacent to HCC were investigated. All patients were HBV-positive. As controls, 5 specimens of HBV-positive cirrhotic liver tissue from patients without liver tumour and 5 specimens of normal liver tissue were investigated.
For routine histological and immunohistological investigation, the tissue was fixed in 40 g/L buffered formaldehyde and embedded in paraffin. Immunohistochemical investigations were performed on all specimens using an undiluted monoclonal mouse antibody against cytokeratin 7 and a polyclonal antibody against albumin (both from DAKO, Glostrup, Dennmark) diluted 1:1 000. Single labelling with each antibody separately, and double labelling using both antibodies on the same section, were carried out.
For conventional transmission electron-microscopic (TEM) investigation, the tissue was fixed in 40 g/L glutaraldehyde in 0.1 mol/L phosphate buffer (pH 7.3). TEM investigations as described elsewhere[5-9] were performed on 10 cases of HLC with associated HCC. In order to distinguish labelling of the 2 antigens (cytokeratin 7 and albumin) in the double labelling procedure, goat anti-mouse IgG, goat anti-rabbit IgG as secondary antibodies and DAB (brown), NBT (green) as chromogen were used respectively.
For ICLSM investigation the specimens from 5 cases of HLC with associated HCC, 2 cases without HCC and 2 specimens of normal liver tissue were fixed in 40 g/L buffered formaldehyde and embedded in paraffin for immunohistochemical investigations.
Paraffined sections were prepared for immunofluorescent labelling. Briefly, primary antibodies against cytokeratin 7 (undiluted) and albumin (1:1 000 diluted in phosphate-buffered saline with 5 g/L bovine serum albumin and 1 g/L gelatine) and secondary antibodies (goat anti-mouse IgG and goat anti- rabbit IgG) conjugated with FITC or Cy3 (Sigma) were used. Double labelling using both antibodies on the same section was performed. Primary antibodies and secondary antibodies were incubated for 1 h at room temperature. Nuclear staining was carried out with DAPI (Sigma) in PBS. Slides were stored at 4 °C and analysed within 24 h. As a control, the primary antibody was omitted.
Immunofluorescence was observed with Zeiss LSM 510 (Zeiss; Jena, Germany). We used an argon laser at 488 nm in combination with a helium neon laser at 543 nm to excite the green (CK7) and red (albumin) fluorochromes simultaneously. Emitted fluorescence was detected with a 505-530 nm bandpass filter for the green signal, a 560 nm longpass filter for the red signal and 633 nm filter for the blue signal.
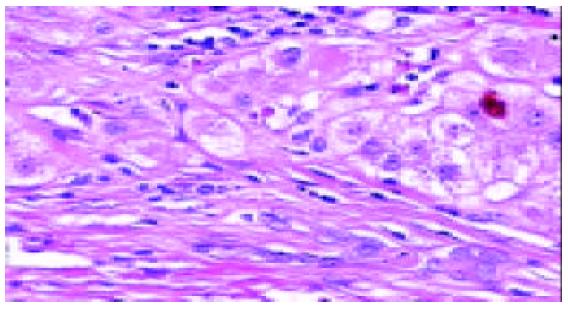
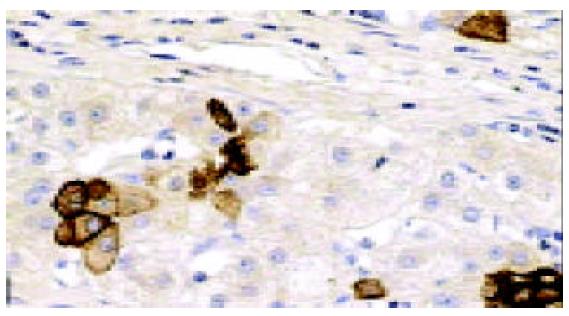
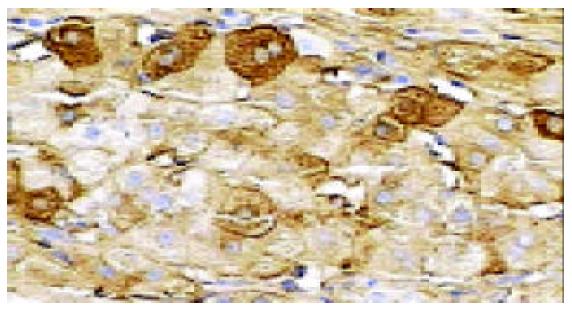
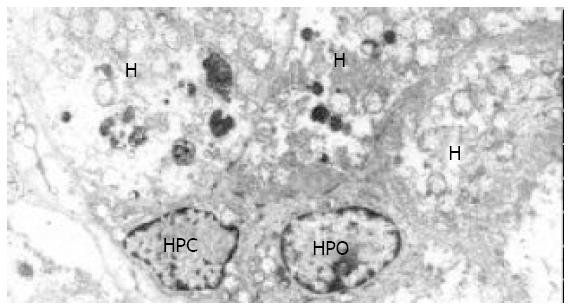
In all the investigated cases of HLC, micronodular and/or macronodular cirrhosis was a basic morphologic feature. In fibrous septae, proliferating bile ductules and lymphocytic infiltrates were found at a varying extent. Hepatic progenitor cells (HPC) with a histological picture morphologically similar to oval cells of rodents were found in all cases studied (Figure 1). HPC were numerous in proliferating bile ductules or noted as discretely scattered cells at the edge of regenerating nodules and in fibrous septae (Figure 1). The number of HPC varied from 2 to 10 in 40 high power fields. Their number was larger in cases exhibiting signs of active regeneration, but no differences were seen between cases with and without associated HCC. HPC, however, were not observed in normal livers. Immunohistochemical investigations showed that HPC could express both cytokeratin 7 and albumin (Figures 2-4). In the normal liver, hepatocytes were immunoreactive for albumin and biliary epithelial cells for cytokeratin 7. No double staining of albumin and cytokeratin 7 could be found in hepatocytes or biliary epithelial cells in normal livers.
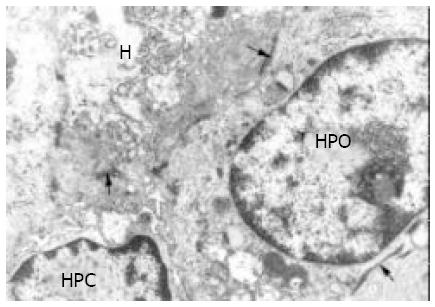
At electron microscopic level, a very small number of cells with ultrastructural features typical of the SEC found in HB[5,6], EBA[8] and HCC[7] were noted in all cases of HLC. As noted by light microscopy, HPC were mainly located in ductules that were composed of 2-8 cells, and they were also seen in the fibrous septae and at the periphery of nodules. They were characterized by their small size (8 to 18 µm), oval shape, scanty cytoplasm with a high nucleus/cytoplasm ratio, tonofilament bundles, tight junctions or desmosome-like junctions, and an oval, electron-dense nucleus in which heterochromatin was seen as small clumps dispersed in the nucleoplasm with peripheral condensation. Junctions were found both between HPC and hepatocytes and between HPC and epithelial cells of small bile ductules (Figures 5 and 6).
The number of organelles in HPC varied. Some contained numerous free ribosomes, and others contained free ribosomes and rough endoplasmic reticulum. As in EBA and hepatoblastoma[8], HPC with ultrastructural features of differentiation towards biliary epithelial cells or hepatocytes were also found in HLC. These differentiated cells were mainly located in proliferating bile ductules. HPC with signs of biliary differentiation contained a higher number of tonofilament bundles than undifferentiated HPC and sometimes exhibited small surface microvilli and formed bile canaliculi with neighboring hepatocytes[9]. Some HPC were partly surrounded by a basement membrane. HPC with signs of hepatocytic differentiation exhibited more cytoplasm, more mitochondria and fewer tonofilament bundles than undifferentiated HLC. Intercellular junctions between HPC and adjacent biliary epithelial cells were noted not only in undifferentiated HLC but also in HLC with signs of biliary and hepatocytic differentiation (Figures 5 and 6).
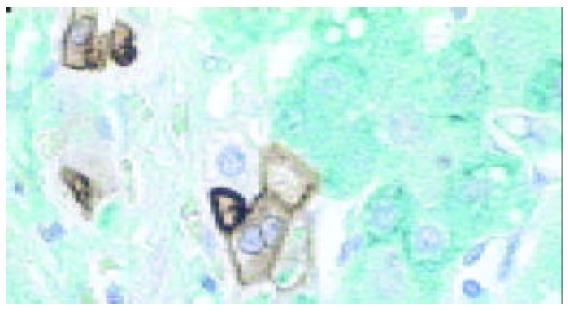
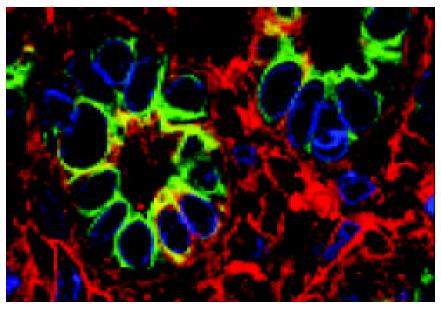
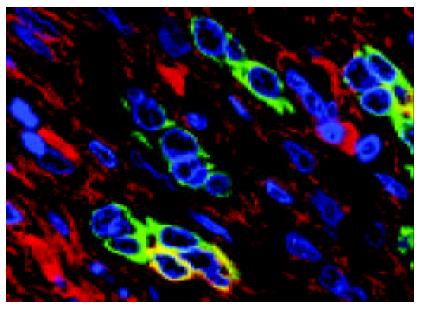
ICLSM investigation revealed labelling of HPC for both cytokeratin 7 and albumin. HPC with double labelling of both cytokeratin 7 and albumin were observed, as noted under light microscopy, at margins of regenerating nodules, in fibrous septae, and especially in proliferating bile ductules that were composed of 2-8 cells. Although we could not see the accurate location of a labelling for cytokeratin 7 (as seen with immunoelectron microscopy, this was associated mainly with tonofilament bundles) and for albumin (this was cytoplasmic and diffuse), but the double labelling in HPC was rarely noted from this method. Some HPC (with signs of biliary differentiation) exhibited a stronger labelling for cytokeratin 7 and a weaker labelling for albumin. In comparison, those cells with signs of hepatocytic differentiation exhibited a stronger labelling for albumin and a weaker labelling for cytokeratin 7 (Figures 7 and 8).
In rodents, administration of hepatocarcinogens could lead to proliferation of oval cells, which are characterized by certain morphological features, such as a small size, an oval shape and a high nucleus/cytoplasm ratio[1-4]. These cells express both biliary and hepatocytic markers, and are able to differentiate into biliary epithelial cells and hepatocytes. It has been widely accepted that oval cells represent hepatic stem cells in rodent livers, or are at least closely related to them[2].
Whether bipotent hepatic stem cells that give rise to both biliary epithelial cells and hepatocytes exist in human livers, however, is still a matter of controversy[2]. Cells with the morphologic features of oval cells in rodents have been recently, described in various liver diseases[11-13]. In previous studies we demonstrated that cells with the morphological and immunophenotypical features of oval cells of rodents, that we termed SEC, existed in human livers under pathological conditions such as in HB[5,6], HCC[7], EBA[8] and HLC[9] but not in normal livers[5-9]. In this study, we further demonstrated with immunohistochemistry, ICLSM and electron microscopic technique, that HPC did occur in HLC due to HBV infection in cases with and without associated HCC.
As in HB, HCC and EBA, HPC in HLC exhibit several important features that closely resemble those of oval cells of rodents. (1) They are often characterized by an oval shape and an oval nucleus, a high nucleus/cytoplasm ratio and a small size with 8-18 µm average diameter. (2) The ultrastructural features of HPC closely resemble those of oval cells as described in rodents[10], i.e., HPC represent desmosome-like or tight junctions, tonofilaments, microvillous interdigitations and basement membrane. (3) They exhibit corresponding immunophenotypes to oval cells as shown by immunohistochemistry and ICLSM. These cells are verified to express both albumin (a marker of hepatocytic differentiation) and cytokeratin 7 (in the liver a marker of biliary differentiation). (4) A positive correlation exists between the number of HPC and the activity of cirrhosis. (5) HPC are located in bile ductules that contain 2-8 cells.
On the basis of our findings in HLC, as described in HB, EBA and HCC, it seems likely that HPC represent potential bipotent stem cells in human livers that by difinition, give rise to biliary epithelial cells and hepatocytes.
There is a likeness of HLC to other liver diseases in that an association exists between the activity or severity of cirrhosis and the number of HPC. This has led us to advance the hypothesis that oval cell proliferation might not be disease-specific but occurs in response to progressive liver injury and fibrosis.
HPC mainly occurred in proliferating bile ductules that were found both in experimental animal models[2] and in various human neoplastic and non-neoplastic liver diseases[11-14]. As to the origin of proliferating ductules, the hypothesis of proliferation of pre-existing bile duct epithelium or a metaplasia of periportal hepatocytes has been reported[2,3,15]. However, there is now increasing evidence that, at least in some cases, proliferating bile ductules arose directly from hepatic progenitor cells not only in rodents[1-4], but also in various human liver diseases[11- 14]. This study indicated again that oval-like cells did appear in proliferating bile ductules in human livers and that these ductules might indeed arise from proliferation and differentiation of hepatic progenitor cells.
HPC have been recently demonstrated only in pathological liver specimens but not in normal livers. It can rationally be speculated that as in rodent livers, the activation and proliferation of putative stem cell pool in human livers took place only if the replicative capacity of hepatocytes was severely impaired after some endogenous or exogenous toxic injury or stimuli[10,13,16]. Several studies indicated that HPC activation in rodent livers was regulated by or associated with various factors, such as tumour necrosis factor-α (TNF-α), transforming factor-α (TGF-α), epidermal growth factor (EGF), hepatocyte growth factor (HGF), etc.[10,13,16]. Some non-hepatocytes such as Kupffer cells and Ito cells have also been reported to participate in HPC activation[3]. These findings await to be verified in human liver diseases.
In conclusion, HLC caused by HBV-infection contain HPC. The findings further support the hypothesis that human hepatic progenitor cells may play a role in the course of liver diseases.
| 1. | Dabeva MD, Petkov PM, Sandhu J, Oren R, Laconi E, Hurston E, Shafritz DA. Proliferation and differentiation of fetal liver epithelial progenitor cells after transplantation into adult rat liver. Am J Pathol. 2000;156:2017-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 117] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Forbes S, Vig P, Poulsom R, Thomas H, Alison M. Hepatic stem cells. J Pathol. 2002;197:510-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 117] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Yin L, Lynch D, Ilic Z, Sell S. Proliferation and differentiation of ductular progenitor cells and littoral cells during the regeneration of the rat liver to CCl4/2-AAF injury. Histol Histopathol. 2002;17:65-81. [PubMed] |
| 4. | Braun KM, Thompson AW, Sandgren EP. Hepatic microenvironment affects oval cell localization in albumin-urokinase-type plasminogen activator transgenic mice. Am J Pathol. 2003;162:195-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Ruck P, Xiao JC, Kaiserling E. Small epithelial cells and the histogenesis of hepatoblastoma. Electron microscopic, immunoelectron microscopic, and immunohistochemical findings. Am J Pathol. 1996;148:321-329. [PubMed] |
| 6. | Ruck P, Xiao JC. Stem-like cells in hepatoblastoma. Med Pediatr Oncol. 2002;39:504-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Xiao JC, Ruck P, Kaiserling E. Small epithelial cells in extrahepatic biliary atresia: electron microscopic and immunoelectron microscopic findings suggest a close relationship to liver progenitor cells. Histopathology. 1999;35:454-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Xiao JC, Ruck P, Kaiserling E. Zur Bedeutung der kleinen epithelialen Zellen beim hepatozellulären Karzinom. Immunohistochemische, elektronenmikroskopische und immunoelektronenmikroskopische Befunde. Verh Dtsch Ges Pathol. 1996;80:407. |
| 9. | Xiao JC, Ruck P, Adam A, Wang TX, Kaiserling E. Small epi-thelial cells in human liver cirrhosis exhibit features of hepatic stem-like cells: immunohistochemical, electron microscopic and immunoelectron microscopic findings. Histopathology. 2003;42:141-149. [RCA] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Kiss A, Schnur J, Szabó Z, Nagy P. Immunohistochemical analysis of atypical ductular reaction in the human liver, with special emphasis on the presence of growth factors and their receptors. Liver. 2001;21:237-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Van Den Heuvel MC, Slooff MJ, Visser L, Muller M, De Jong KP, Poppema S, Gouw AS. Expression of anti-OV6 antibody and anti-N-CAM antibody along the biliary line of normal and diseased human livers. Hepatology. 2001;33:1387-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Tan J, Hytiroglou P, Wieczorek R, Park YN, Thung SN, Arias B, Theise ND. Immunohistochemical evidence for hepatic progenitor cells in liver diseases. Liver. 2002;22:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Libbrecht L, Roskams T. Hepatic progenitor cells in human liver diseases. Semin Cell Dev Biol. 2002;13:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 148] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Crosby HA, Kelly DA, Strain AJ. Human hepatic stem-like cells isolated using c-kit or CD34 can differentiate into biliary epithelium. Gastroenterology. 2001;120:534-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 167] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Zhang Y, Bai XF, Huang CX. Hepatic stem cells: existence and origin. World J Gastroenterol. 2003;9:201-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Lowes KN, Croager EJ, Olynyk JK, Abraham LJ, Yeoh GC. Oval cell-mediated liver regeneration: Role of cytokines and growth factors. J Gastroenterol Hepatol. 2003;18:4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 119] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
Edited by Lu HM and Wang XL Proofread by Xu FM