Published online Apr 15, 2004. doi: 10.3748/wjg.v10.i8.1137
Revised: December 10, 2003
Accepted: December 24, 2003
Published online: April 15, 2004
AIM: To report the results of radiofrequency ablation (RFA) of hepatocellular carcinoma (HCC) in cirrhotic patients and to describe the treatment related complications (mainly the rapid intrahepatic neoplastic progression).
METHODS: Eighty-seven consecutive cirrhotic patients with 104 HCC (mean diameter 3.9 cm, 1.3 SD) were submitted to RFA between January 1998 and June 2003. In all cases RFA was performed with percutaneous approach under ultrasound guidance using expandable electrode needles. Treatment efficacy (necrosis and recurrence) was estimated with dual phase computed tomography (CT) and alpha-fetoprotein (AFP) level.
RESULTS: Complete necrosis rate after single or multiple treatment was 100%, 87.7% and 57.1% in HCC smaller than 3 cm, between 3 and 5 cm and larger than 5 cm respectively (P = 0.02). Seventeen lesions of 88(19.3%) developed local recurrence after complete necrosis during a mean follow up of 19.2 mo. There were no treatment-related deaths in 130 procedures and major complications occurred in 8 patients (6.1 %). In 4 patients, although complete local necrosis was achieved, we observed rapid intrahepatic neoplastic progression after treatment. Risk factors for rapid neoplastic progression were high preoperative AFP values and location of the tumor near segmental portal branches.
CONCLUSION: RFA is an effective treatment for hepatocellular carcinoma smaller than 5 cm with complete necrosis in more than 80% of lesions. Patients with elevated AFP levels and tumors located near the main portal branch are at risk for rapid neoplastic progression after RFA. Further studies are necessary to evaluate the incidence and pathogenesis of this underestimated complication.
- Citation: Ruzzenente A, Manzoni G, Molfetta M, Pachera S, Genco B, Donataccio M, Guglielmi A. Rapid progression of hepatocellular carcinoma after Radiofrequency Ablation. World J Gastroenterol 2004; 10(8): 1137-1140
- URL: https://www.wjgnet.com/1007-9327/full/v10/i8/1137.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i8.1137
Hepatocellular carcinoma (HCC) is one of the most frequent solid tumors with a worldwide incidence between 250 000 and 1 000 000 new cases per year[1,2].
Surgical therapies could be applied only in 10-20% of patients[3,4]. Liver resection is applicable in a small portion of patients because of advanced neoplastic stage or severity of liver diseases. Liver transplantation is available only in a small number of patients for shortage of donors[5].
The majority of patients, ineligible for surgical therapies, can be submitted to local ablative therapies like percutaneous ethanol injection (PEI) and radiofrequency ablation (RFA). These local ablative therapies were considered to be radical in small HCCs[6].
RFA showed good control of tumors with necrosis in more than 90% of HCC smaller than 5 cm[7-10]. In HCC larger than 5 cm results were unsatisfactory with complete necrosis in less than 30%[11].
Frequency of major complications related to the procedure was low, ranging from 5% to 15%[12,13]. Self-limiting intraperitoneal bleeding, liver abscess and right pleural effusions have been the most frequently reported complications[7,14].
Tumor seeding along the needle tract was also described but its incidence ranging from 0.6% to 12%, has been a matter of debate[15-18].
Rapid intrahepatic neoplastic progression after treatment was described in literature only in 1 case report after transcatheter arterial chemoembolization (TACE) and RFA[19].
To our knowledge no authors described this complication after RFA treatment.
The aim of this study was to describe the treatment related complications (mainly the rapid intrahepatic neoplastic progression) and the response rate of RFA of HCC in cirrhosis.
From January 1st, 1998 to June 1st, 2003 patients submitted to RFA at the 1st Department of Surgery of Verona University Medical School were included in this study. Before treatment a detailed description of procedure was provided to patients and written informed consent was obtained from all cases. HCC was diagnosed on the basis of radiological criteria (2 coincident imaging techniques) or combined criteria [1 imaging technique associated with elevated alpha-fetoprotein (AFP) levels] according to Barcellona EASL Conference[6]. Fine needle biopsy was performed only in cases of uncertain diagnosis[6]. Before undergoing the procedure, patients underwent baseline clinical and laboratory evaluation of hepatic function, coagulation profile, blood cells and platelet count, hepatitis B and C virus serology and serum AFP level. Severity of cirrhosis was classified according to Child-Pugh classification and CLIP score[20,21]. Patients eligible for RFA with Child-Pugh B cirrhosis and/or multinodular tumors, and small number of patients with Child-Pugh A cirrhosis and single potentially resecable tumor were included in the study.
Exclusion criteria from study were extra-hepatic metastasis, more than 4 nodules, tumor diameter > 7 cm, Child-Pugh C cirrhosis, severe ascites, severe coagulopathy (platelets count < 40 000/mmc and PT > 1.5 INR) if not corrected with medical therapy and fresh-frozen plasma.
Antibiotic prophylaxis was administered in all patients before treatment. All patients were treated in operating room under general anesthesia. Treatment was conducted in all patients with percutaneous approach under ultrasound guidance using a radiofrequency generator (RITA medical system model 500 and 1 500, Mountain View, CA) through an expandable electrode needle as previously described[22]. Blood count and liver function tests were performed 12 and 24 h after RFA.
All patients were discharged after 1 d, unless complications necessitated a longer hospitalization.
Treatment results were estimated by dual-phase computed tomography (CT) and AFP level performed 30 d after the procedure. CT and serum AFP were performed every 3 mo for the first year and then every 6 mo.
At dual phase, CT complete necrosis was defined as the absence of pathologic enhancement within or at the periphery of the treated HCC after at least 2 consecutive CT examinations.
Incomplete necrosis or local recurrences of HCC detected during follow up were retreated with RFA or PEI.
Data were collected prospectively and analyzed with statistical software (SPSS inc., Chicago, IL). Comparisons between different categories were carried out with contingency tables, and significance was determined by χ2. For all comparisons, significance was set at 0.05.
From January 1st , 1998 to June 1st , 2003, 87 patients with 104 HCCs and liver cirrhosis were submitted to RFA. Mean age was 67.9 years (range 41-88 years). Patient characteristics are described in Table 1. The mean follow up time was 19.2 mo. After the first treatment complete necrosis was achieved in 76 of 104 HCCs (73.1%). In 28 HCCs with incomplete necrosis RFA was repeated. After multiple treatments (range 2-4) 12 of 28 HCCs, with incomplete necrosis were completely ablated. Complete necrosis was achieved in HCC smaller than 3 cm, between 3 cm and 5 cm and bigger than 5 cm in 100%, 87.7% and 57.1%, respectively (P = 0.02). The elevated AFP level before treatment was decreased to normal levels after complete necrosis. During the follow up 17 of 88 HCCs (19.3%) with complete necrosis showed local recurrence and 30 of 87 patients (34.4%) developed new intrahepatic tumors.
| Characteristic | n | (%) |
| Gender | ||
| Male | 71 | 81.6 |
| Female | 16 | 18.4 |
| Ethiology of cirrhosis | ||
| HCV related | 46 | 52.9 |
| HBV related | 11 | 12.6 |
| HBV+HCV | 3 | 3.4 |
| Alcoholic | 24 | 27.7 |
| Other causes | 3 | 3.4 |
| Child-Pugh classification | ||
| A | 48 | 55.2 |
| B | 39 | 44.8 |
| C | 0 | 0 |
| CLIP score | ||
| 0 | 25 | 28.7 |
| 1 | 42 | 48.3 |
| 2 | 19 | 21.8 |
| 4 | 1 | 1.2 |
| Number of lesions | ||
| Single | 53 | 60.9 |
| Multiple | 34 | 39.1 |
| Morphology of HCC | ||
| Infiltrating | 18 | 17.3 |
| Noninfiltrating | 86 | 82.7 |
| Alfa-fetoprotein level | ||
| < 200 kU/L | 79 | 90.8 |
| 200 kU/L | 8 | 9.1 |
In 87 patients, 130 procedures were performed and treatment related complications occurred in 22 cases (16.9%) (Table 2). Minor complications occurred in 13 cases: fever > 38 °C and moderate pain for 5-6 d in 10 cases, right pleural effusions in 2 cases, cutaneous burn in the insertion site of the neddle in 1 case. Major complications were observed in 8 patients: rapid tumor intrahepatic progression in 4 cases, bacterial endocarditis in 1 case, needle track seeding in 1 case, intraperitoneal bleeding in 1 case, hepatic decompensation in 1 case. All treatment related complications were managed with medical therapy. No treatment related mortality was observed.
| Events n (%) | |
| Minor complications | 13 (10) |
| Fever (> 38 °C) and moderate pain (5-6 d) | 10 (7.7) |
| Right pleural effusion | 2 (1.5) |
| Cutaneous burn | 1 (0.8) |
| Major complications | 8 (6.1) |
| Rapid tumor progression | 4 (2.9) |
| Bacterial endocarditis | 1 (0.8) |
| Neoplastic seeding | 1 (0.8) |
| Peritoneal bleeding | 1 (0.8) |
| Hepatic decompensation | 1 (0.8) |
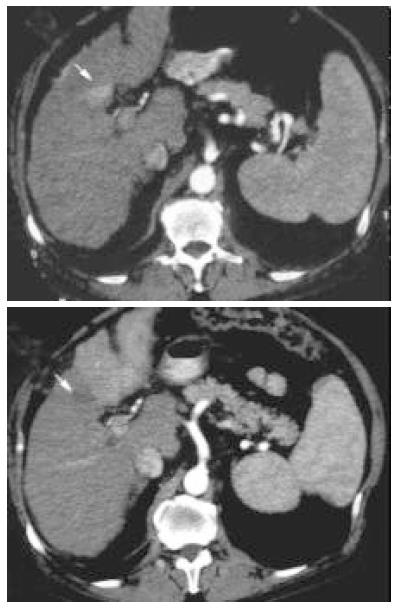
After treatment, CT examination showed tumor complete necrosis with a wide neoplastic spread to the adjacent liver segments (Figure 1). After 30 d, AFP values showed a rapid increase in 3 of 4 patients, from a pretreatment value of 119 kU/L, 560 kU/L and 1 313 kU/L to 4 997 kU/L, 2 500 kU/L and 5 282 kU/L, respectively. In the fourth patient, the baseline AFP value of 69 kU/L did not increase. Characteristics of patients and tumors are described in Table 3. We analyzed 10 variables in order to identify the risk factors for rapid tumor progression. The high AFP level and location of the tumor near the primary or secondary portal vein branches were more representative variables (Table 4). During the follow up 2 patients died after 2 and 3 mo for tumor progression, two patients were alive after 11 and 7 mo and they were submitted to transcatheter arterial chemoembolization (TACE).
| Gender, age, etiology | Tumor size (cm) | Location | Differentiation | |
| 1. Male, 66 yr, alcohol | 3.5 | V segment | Near right portal trunka | Poor |
| 2.Male, 75 yr, HCV | 3.8 | VI segment | Near segmental portal brancha | Poor |
| 3. Male, 59 yr, HBV, HCV | 4.5 | VII segment | Near cava vein | Poor |
| 3.Male, 62 yr, HBV | 4.5 | IV segment | Near segmental portal brancha | Moderate |
| Variables | No tumor progression (83) | Tumor progression (4) | P value |
| Child-Pugh class | |||
| A/B | 45/38 | 3/1 | 0.41 |
| CLIP score | |||
| 0/1/2/4 | 25/40/17/1 | 0/2/2/0 | 0.43 |
| Number lesions | |||
| Single/multiple | 50/33 | 2/2 | 0.68 |
| AFP value | |||
| < 200/≥ 200 kU/L | 77/6 | 2/2 | 0.04 |
| Tumor size | |||
| < 3 cm/3-5 cm/5-7 cm | 10/59/14 | 0/4/0 | 0.45 |
| Tumor morphology | |||
| Infiltrating/ | 15/68 | 1/3 | 0.72 |
| noninfiltrating | |||
| Portal vein thrombosis | |||
| No/main portal | 71/1/3 | 3/0/1 | 0.17 |
| branch/sectorial | |||
| Tumor location | |||
| Peripheral/near | 60/23 | 1/3 | 0.04 |
| main portal | |||
| Treatment | |||
| Duration RFA (min) | 27.7±13.7 | 25.6±7.5 | 0.40 |
| Number insertions | 2.0±1.3 | 1.7±0.9 | 0.33 |
More than 80% of patients with HCC could not be submitted to surgical treatment due to advanced tumor stage and poor hepatic function[3,4]. In selected patients ablative treatments could achieve good results, which were similar to those of surgical resection in small HCCs[23,24]. RFA could achieve complete necrosis in 90% of lesions smaller than 5 cm[7-10,22]. In our study local recurrence occurred in 19.3%of tumors with complete necrosis and it was related to tumor size. There were no local recurrences during the follow up for HCCs smaller than 3 cm. In early clinical results RFA showed low incidence of major complications (5-15%)[12,13,25]. Most frequent complications reported in literature were: capsular necrosis, intraperitoneal hemorrhage (usually self-limiting), subcapsular hematoma, cholecistitis, hepatic abscesses[7,14]. Needle track seeding after RFA was reported with low incidence (0.6-2.8%), but in a recent study in 32 patients Llovet et al reported an incidence of 12%[15-18]. The authors identified that tumors with poor differentiation, subcapsular location and high AFP levels were at risk for needle track seeding[15].
Another complication recently observed was the rapid tumor progression after local treatment[19]. This complication after RFA was described in only one case report in a patient who was previously submitted to TACE. In our experience 4 patients (4.5%) showed rapid intrahepatic spread of HCC after RFA without extrahepatic metastasis. To our knowledge this is the first report that describes this type of complication after RFA. Seki et al, in their case report, suggested that aggressive biological behavior of the tumor might be involved in tumor progression[19]. Moreover the author suggested that neoplastic cell spreading might have been promoted by TACE with selection of highly malignant cells with low adhesive potential after partial necrosis.
Our paper describes the occurrence of rapid neoplastic spread after RFA and we have identified the possible risk factors for this complication. Assessment of tumor and patients characteristics showed that high AFP level and location of tumor near the portal vein branches were associated with this complication.Moreover 3 of 4 patients had a poor differentiation of the tumor.
In our opinion, mechanisms involved in the pathogenesis of neoplastic progression may be the following. a) Tumors with vascular invasion are characterized by elevated intratumoral pressure[26]. RFA during energy application may increase intratumoral pressure and favor intravascular spread of the tumor. b) Hooks delivering in “umbrella type” expandable needles may promote migration of neoplastic cells into portal vein branches. c) Creation of arterovenus fistula was reported after RFA[12,27]. This alteration of hepatic vascularization may promote migration of neoplastic cells into portal vein circulation.
Definitive conclusions cannot be drawn because this complication is probably still underestimated. In our opinion, tumors with a high AFP level, location near the main portal branches and poor differentiation should be carefully evaluated before RFA treatment.
| 1. | Simonetti RG, Liberati A, Angiolini C, Pagliaro L. Treatment of hepatocellular carcinoma: a systematic review of randomized controlled trials. Ann Oncol. 1997;8:117-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 280] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 2. | Schafer DF, Sorrell MF. Hepatocellular carcinoma. Lancet. 1999;353:1253-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 400] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 3. | Farinati F, Rinaldi M, Gianni S, Naccarato R. How should patients with hepatocellular carcinoma be staged? Validation of a new prognostic system. Cancer. 2000;89:2266-2273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 921] [Article Influence: 34.1] [Reference Citation Analysis (2)] |
| 5. | Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 857] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 6. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodes J. EASL Panel of Experts on HCC. Clinical management of hepato-cellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3252] [Cited by in RCA: 3258] [Article Influence: 130.3] [Reference Citation Analysis (1)] |
| 7. | Curley SA, Izzo F, Ellis LM, Nicolas Vauthey J, Vallone P. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232:381-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 530] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 8. | Gazelle GS, Goldberg SN, Solbiati L, Livraghi T. Tumor ablation with radio-frequency energy. Radiology. 2000;217:633-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 501] [Article Influence: 19.3] [Reference Citation Analysis (3)] |
| 9. | Giovannini M, Moutardier V, Danisi C, Bories E, Pesenti C, Del pero JR. Treatment of Hepatocellular Carcinoma Using Percuta-neous Radiofrequency Thermoablation: Results and Outcomes in 56 Patients. J Gastrointest Surg. 2003;7:791-796. [RCA] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 10. | Shibata T, Iimuro Y, Yamamoto Y, Maetani Y, Ametani F, Itoh K, Konishi J. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002;223:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 393] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 11. | Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, Gazelle GS. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 806] [Cited by in RCA: 742] [Article Influence: 28.5] [Reference Citation Analysis (1)] |
| 12. | Mulier S, Mulier P, Ni Y, Miao Y, Dupas B, Marchal G, De Wever I, Michel L. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002;89:1206-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 497] [Article Influence: 20.7] [Reference Citation Analysis (1)] |
| 13. | de Baère T, Risse O, Kuoch V, Dromain C, Sengel C, Smayra T, Gamal El Din M, Letoublon C, Elias D. Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR Am J Roentgenol. 2003;181:695-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 331] [Article Influence: 14.4] [Reference Citation Analysis (1)] |
| 14. | McGhana JP, Dodd GD. Radiofrequency ablation of the liver: current status. AJR Am J Roentgenol. 2001;176:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 408] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 15. | Llovet JM, Vilana R, Brú C, Bianchi L, Salmeron JM, Boix L, Ganau S, Sala M, Pagès M, Ayuso C. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 530] [Article Influence: 21.2] [Reference Citation Analysis (1)] |
| 16. | Bolondi L, Gaiani S, Celli N, Piscaglia F. Tumor dissemination after radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2001;34:608. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 17. | de Sio I, Castellano L, De Girolamo V, di Santolo SS, Marone A, Del Vecchio Blanco C, Marone G. Tumor dissemination after radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2001;34:609-10; author reply 610-1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Goldberg SN, Solbiati L. Tumor dissemination after radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2001;34:609; author reply 610-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 19. | Seki T, Tamai T, Ikeda K. Rapid progression of hepatocellular carcinoma after transcatheter arterial chemoembolization and percutaneous radiofrequency ablation in the primary tumour region. Eur J Gastroenterol Hepatol. 2001;13:291-294. [RCA] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 2.5] [Reference Citation Analysis (2)] |
| 20. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5821] [Article Influence: 109.8] [Reference Citation Analysis (2)] |
| 21. | A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 967] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 22. | Guglielmi A, Ruzzenente A, Battocchia A, Tonon A, Fracastoro G, Cordiano C. Radiofrequency ablation of hepatocellular carcinoma in cirrhotic patients. Hepatogastroenterology. 2003;50:480-484. [PubMed] |
| 23. | Vivarelli M, Guglielmi A, Ruzzenente A, Cucchetti A, Bellusci R, Cordiano C, Cavallari A. Surgical resection versus percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma on cirrhotic liver. Ann Surg. 2004;240:102-107. [PubMed] |
| 24. | Arii S, Yamaoka Y, Futagawa S, Inoue K, Kobayashi K, Kojiro M, Makuuchi M, Nakamura Y, Okita K, Yamada R. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology. 2000;32:1224-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 539] [Article Influence: 20.7] [Reference Citation Analysis (10)] |
| 25. | Jiang HC, Liu LX, Piao DX, Xu J, Zheng M, Zhu AL, Qi SY, Zhang WH, Wu LF. Clinical short-term results of radiofrequency ablation in liver cancers. World J Gastroenterol. 2002;8:624-630. [PubMed] |
| 26. | Tanaka T, Yamanaka N, Oriyama T, Furukawa K, Okamoto E. Factors regulating tumor pressure in hepatocellular carcinoma and implications for tumor spread. Hepatology. 1997;26:283-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Catalano O, Esposito M, Nunziata A, Siani A. Multiphase helical CT findings after percutaneous ablation procedures for hepatocellular carcinoma. Abdom Imaging. 2000;25:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Edited by Wang XL and Xu FM