INTRODUCTION
Liver transplantation has become an accepted therapy for end-stage liver diseases, but poor immediate graft function presents a persistent problem and contributes to mortality rates[1-5]. The clinical incidence of primary graft nonfunction is strongly dependent on the duration of cold ischemia storage[6]. It has become increasingly evident that reperfusion after ischemia is responsible for the majority of tissue injuries in liver transplantation[5-7]. In addition, recent evidence confirms that liver ischemia-reperfusion injury is a result of the activation of inflammatory mediators in the early phase of reperfusion[8-11].
Nuclear factor-κB (NF-κB) regulates the expression of many genes in which early response products are critical for the development of acute inflammation[12-14]. But NF-κB appears to have both beneficial and harmful effects on liver transplantation[15]. It promotes liver regeneration and prevents apoptosis[23-25], but may also contribute to the inflammatory response to ischemia/reperfusion. NF-κB acts on target genes for proinflammatory cytokines, chemokines, and cell adhesion molecules, thus mediating inflammatory responses. In addition, products of the genes regulated by NF-κB often cause the activation of NF-κB, which creates a positive feedback loop that may amplify and perpetuate local inflammatory responses[14]. But the exact role of NF-κB activity in liver transplantation would depend on the regeneration and apoptosis or ischemia/reperfusion injury, which is the main factor leading to hepatocyte injury after transplantation. However, no definitive evidence has been found for the role of NF-κB activation and inflammatory response after liver transplantation, especially in relation to cold-ischemia time.
In the current study, we investigated the importance of NF-κB in a survival rat model of orthotopic liver transplantation. We determined the time of NF-κB activation after OLT in relation to the expression of tumor necrosis factor (TNF-α), intercellular adhesion molecule (ICAM-1), and cytokine-induced neutrophil chemoattractant (CINC), as well as the recruitment and activation of neutrophils in the grafted livers, with varying cold ischemia time after liver transplantation, to determine whether prolonged cold ischemia time promoted NF-κB activation and inflammatory response in the grafts after reperfusion.
MATERIALS AND METHODS
Experimental protocol
Male Wistar rats weighing 200-250 g were purchased from the Animal Experimental Center, Nanjing Military Command. Animals were allocated randomly to 3 groups (n = 30 in each group) according to the cold ischemia time for 6 h, 18 h, and 24 h. Liver harvesting and orthotopic transplantation were performed using the method described by kamada and Calne[16] with minor modifications[17], and the hepatic artery was not reconstructed. The portal vein clamping time in the recipient varied from 18 to 20 min, implantation surgery required less than 50 min, all the procedures took about 60 min. No significant difference was seen in portal clamping time between groups. For the survival study, 8 rats in each group were examined. Animals that survived more than 7 d were considered survivors. The recipients were sacrificed at 0 h, 0.5 h, 1 h, 2 h, 4 h, and 6 h after reperfusion. Blood samples were collected from subhepatic vena cava to determine the serum levels of asparate aminotransferase (ALT) and neutrophil activity at indicated time point. The median lobe of the liver was carefully excised at the designated time point and stored at -80 °C for analysis.
NF-κB activity evaluation
Nuclear extracts were prepared using a nuclear extract kit (Active Motif) and p65/relA subunit of NF-κB was determined using a TransAM NF-κB p65 kit (Active Motif) according to the manufacturer’s protocols. The concentration of p65/relA subunit in liver tissue homogenates was standardized as the total nuclear protein content in each specimen measured by the Pierce BCA protein assay reagent (Jiancheng, Nanjing, China). NF-κB activity was expressed as p65/relA subunit ug/mg total nuclear protein.
Measurement of TNF-α, CINC and ICAM-1
Hepatic levels of TNF-α, CINC and ICAM-1 were determined as previously described[18]. In brief, the medial hepatic lobe was weighed and then homogenized in 5 mL of 0.1 mol/L phosphate buffer (pH 7.4) containing 0.5 g/L of sodium azide at 4 °C. Homogenate was first centrifuged at 2000 g for 10 min to remove solid tissue debris. Supernatant was assayed using a rat ELISA system (Amersham, Buckinghamshire, UK). Concentration of antigens in liver tissue homogenates was standardized as the total protein content in each specimen measured by the Pierce BCA protein assay reagent. The results were expressed as pg/mg total protein.
Intrahepatic neutrophil accumulation assessment
Activity of MPO, an enzyme stored in the azurophilic granules of neutrophils, has been used as a well-established marker to determine tissue neutrophil sequestration[19]. Frozen lungs were thawed and extracted for MPO, following the homogenization and sonication procedure as described in manufacturer’s protocols (Jiancheng,Nanjing, China). MPO activity in the supernatant was measured and calculated from the absorbance (at 460 nm) changes resulting from decomposition of H2O2 in the presence of o-dianisidine and was expressed as units per milligram wet weight of tissue. To control intravascular leukocytosis, we calculated the ratio of MPO activity in tissue to WBC count in peripheral blood, and expressed it as MPO/WBC.
Circulating neutrophil activity analysis
Heparinized blood samples from each animal collected at each time point were prepared for flow cytometric analysis[20]. For CD11b and L-selectin determination, 50 μL of samples was incubated with 10 μL of PE-labeled mouse anti-rat L-selectin antibody (Camarillo, CA) and 10 μL of FITC-labeled mouse anti-rat CD11b antibody (Camarillo, CA) for 15 min, then 1 mL of FACS lysing solution (Becton Dickinson, San Jose, CA) was added 10 min, and then centrifuged at 1 500 g for 5 min. The cell pellets were washed, then resuspended in 500 μL of PBS. The cells were read on a Becton-Dickinson FACS Calibur
Statistical analysis
The data were expressed as mean ± SD. Statistical analysis was performed using analysis of variance on SPSS software (version 11.0 for windows), evaluated by ANOVA, and followed by S-N-K multiple comparisons. A P value less than 0.05 was considered statistically significant.
RESULTS
Survival study
All recipients recovered from anesthesia within 15 min, no survivor died within 24 h after reperfusion and experienced massive ascites at the time of death in the absence of technical complication. Thus, the cause of death after reperfusion was considered to be the primary graft nonfunction (PGNF). In all groups, their conditions rapidly deteriorated within 2 h to 4 h after surgery. In the 6 h cold-ischemia group, the general situation was much better, all recipients survived (100%) for more than 7 d. In the 18 h cold-ischemia group, 5 of 8 recipients survived (62.5%) for more than 7 d. In the 24 h cold-ischemia group, no recipient survived (0%) for more than 7 d.
Evaluation of postransplantation liver function time
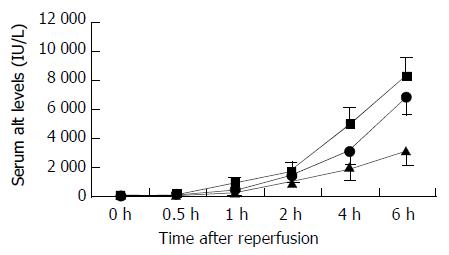
Serum ALT levels increased at 0.5 h after reperfusion, and remained greatly elevated throughout 6 h reperfusion, increased significantly in the 18 h and 24 h cold-preserved groups, as compared with those in the 6 h group during 6 hours’ observation (Figure 1).
Figure 1 Changes in serum ALT levels after reperfusion.
Serum ALT levels significantly increased in the 24 h (closed square) and 18 h (closed circle) cold-preserved groups, compared with the 6 h group (closed triangle) within 0.5 h after reperfusion (P < 0.05, respectively). Results are mean ± SD for each time point in each group (n = 6).
NF-κB activation time
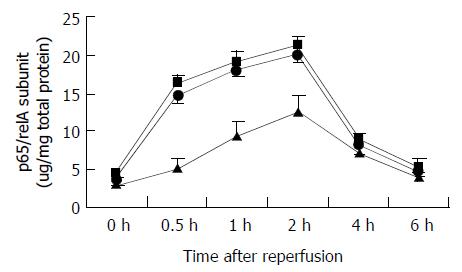
The time of NF-κB activity in liver tissue was established by evaluating p65/relA subunit in nuclear extracts from the whole liver obtained at various time points. In three groups, the activity of NF-κB in the preserved grafts increased slightly at 0.5 h after reperfusion, increased markedly at 2 h after reperfusion and decreased by 4 h (Figure 2). P65/relA subunit significantly increased with prolonged cold preservation, especially at 0.5 h and 1 h after reperfusion. In the 24 h cold-preserved group, p65/relA subunit increased to 3.2 fold of that in the 6 h cold-preserved group at 0.5 h after reperfusion. In the 24 h cold-preserved group, p65/relA subunit increased to 1.7 fold of that in the 6 h cold-preserved group at 2 h after reperfusion. Furthermore, prolonged cold preservation had no effect on p65/relA subunit at 0 h time point, but changed p65/relA subunit level at 6 h after reperfusion, though it had no significant difference (Figure 2).
Figure 2 Changes in p65/relA subunit levels after reperfusion.
P65/relA subunit levels significantly increased in the 24 h (closed square) and 18 h (closed circle) cold-preserved groups, compared with the 6 h group (closed triangle) at 0.5 h, 1 h, and 2 h after reperfusion (P < 0.05, respectively). Results are mean ± SD for each time point in each group (n = 6).
Inflammatory mediator expression time
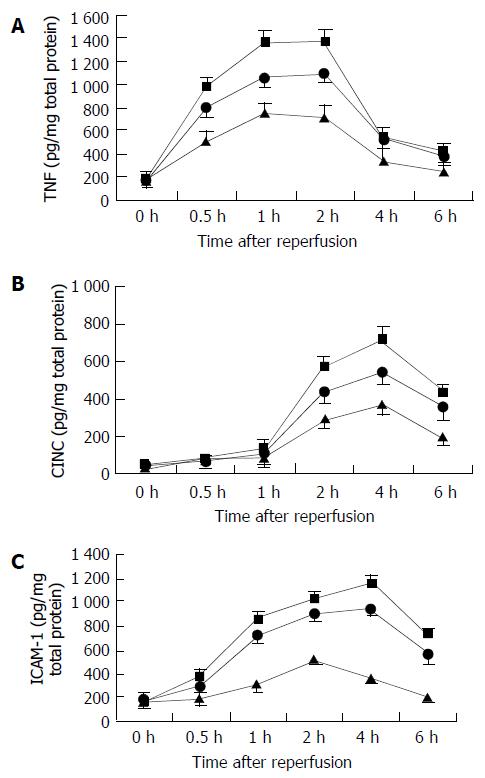
We determined the time of OLT-induced TNF-α, CINC, and ICAM-1 expression in the same tissues as used for NF-κBp65 transcription factor assay. Increases of TNF-α expression were maximal at 1 to 2 h after reperfusion, whereas CINC expression reached the peak at 4 h, and ICAM-1expression peaked at 2 to 4 h (Figure 3). CINC and ICAM-1 were preceded by NF-κB activation (peaked at 2 h after reperfusion). CINC and ICAM-1 expression, especially TNF-α, were significantly increased in the 18 h and 24 h cold-preserved groups compared with the 6 h cold-ischemia group. Prolonged cold preservation did not affect the time of inflammatory mediator response after OLT.
Figure 3 Changes in intrahepatic inflammatory mediator responses after reperfusion.
A: TNF-α levels, B: CINC levels, C: ICAM-1 levels. TNF-α levels significantly increased in the 24 h and 18 h groups 0.5 h after reperfusion, compared with the 6 h group (P < 0.05, respectively). CINC levels significantly in-creased in the 24 h and 18 h groups 2 h after reperfusion, com-pared with the 6 h group (P < 0.05, respectively). ICAM-1 levels significantly increased in the 24 h and 18 h cold-preserved groups 1 h after reperfusion, compared with the 6 h group (P < 0.05, respectively). Results are mean ± SD for each time point in each group (n = 6). 6 h group—closed triangle, 24 h group— closed square, and 18 h group—closed circle.
Neutrophils sequestration time in graft
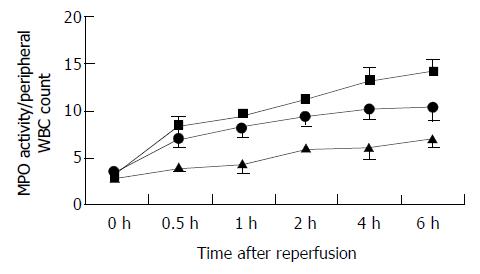
We utilized the MPO biochemical assay to measure tissue neutrophil infiltration over the course of reperfusion. In all recipients, MPO/WBC was significantly elevated within 30 min of reperfusion and remained at significantly elevated level 4 h after of reperfusion (Figure 4). Prolonged cold preservation promoted neutrophil accumulation.
Figure 4 Changes in accumulation of neutrophils in the graft after reperfusion, as assessed by MPO/WBC.
MPO/WBC in the 24 h (closed square) and 18 h (closed circle) cold-preserved groups increased significantly, compared with the 6 h group (closed triangle) within 0.5 h after reperfusion (P < 0.05, respectively). Results are mean ± SD for each time point in each group (n = 6).
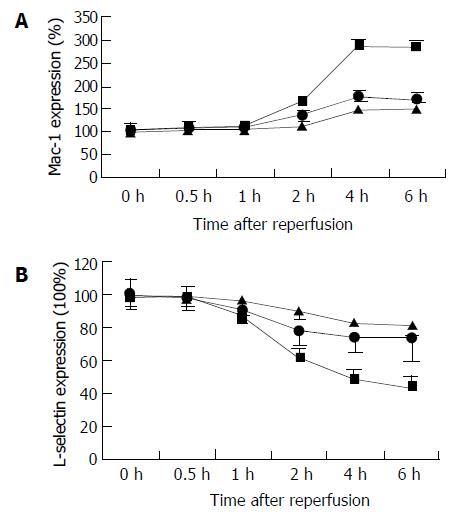
Time of circulating neutrophil activity after reperfusion
Increased surface expression of Mac-1 (CD11b/CD18) is important to neutrophil adherence to parenchyma cells. CD11b content of circulating neutrophils was quantitatively evaluated by flow cytometric analysis at selected time points after the onset reperfusion (Figure 5). In the 24 h cold-preserved group, expression of Mac-1 at the control time point (0 h) showed low levels of CD11b (mean fluorescence intensity was 76.75 ± 19.15), which reached 131% at 2 h, and above the baseline levels of 227% at 4 h, and remained constantly elevated 6 h after reperfusion. Flow cytometric analysis of circulating neutrophils indicated that prolonged cold ischemia had no effect on the time of its CD11b expression, but increased the expression level. In the 6 h cold-preserved group, expression of Mac-1 reached 147% of baseline at 4 h after reperfusion. At the same time, L-selectin was shed, resulting in 41% loss of the adhesion molecules at 4 h in the 24 h cold-preserved group.
Figure 5 Changes in circulating neutrophil activity in the recipents after reperfusion, as assessed by CD11b and L-selectin expression.
A: CD11b expression, B: L-selectin expression. CD11b expression elevated significantly in the 24 h (closed square) and 18 h (closed circle) groups, compared with the 6 h group (closed triangle) 2 h after reperfusion (P < 0.05, respectively). L- selectin shed significantly in the 24 h (closed square) and 18 h (closed circle) groups, compared with the 6 h group (closed triangle) 1 h after reperfusion (P < 0.05, respectively). Results are the mean ± SD for each time point in each group (n = 6).
DISCUSSION
Graft injury after reperfusion has been one of the critical problems to be overcome in the field of organ transplantation[1-7]. During recent years, many cellular and molecular events mediating graft inflammatory injury after OLT have been clarified[8-11]. NF-κB has been found to be an amplifying and perpetuating mechanism that can exaggerate the disease-specific inflammatory process through the coordinated activation of many inflammatory genes[13,14,22]. However, there has not been definitive evidence of what a role it played in NF-κB activation and inflammatory response in relation to graft injury and cold-preservation time after liver transplantation.
In this study, we extended our investigation on the pathogenesis of neutrophilic graft inflammation following rat orthotopic liver transplantation to assess the potential contribution of the transcription factor NF-κB. Since transcription factors determine cellular phenotype by specifying protein production, it is particularly interesting to relate changes in activation of transcription factors such as NF-κB, to the expression of specific proteins and evolution of biological relevant end points[23]. We studied the expression of TNF-α, ICAM-1, and CINC because of their critical roles in the reperfusion-induced inflammatory response. In the rat model of orthotopic liver transplantation, we have shown a link between NF-κB activation, intrahepatic TNF-α, CINC and ICAM-1 expression, neutrophilic inflammation, and graft injury. We further demonstrated that reperfusion-induced NF-κB activation in vivo preceded the expression of TNF-α, CINC, and ICAM-1. In addition, we have shown that prolonged cold preservation would promote NF-κB activation and inflammatory response in the graft. These observations support the hypothesis that elevated inflammatory response in the extended cold-preserved liver might be involved in NF-κB activation and contribute to subsequent neutrophil-mediated tissue injury after liver transplantation.
In this study, the expression of CINC and ICAM-1 in the graft was at a detectable level even in nonreperfusion rats (0 h time point), suggesting that CINC and ICAM-1proteins might be constitutively expressed in the liver. However, after cold ischemia/reperfusion, intrahepatic CINC and ICAM-1 increased with the duration of cold-ischemia. In contrast, intrahepatic TNF-α was not detectable before reperfusion, implicating that TNF-α was an inducible chemokine in the liver. However, a significant increase of prominent up-regulation of intrahepatic TNF-α was found in the 24 h cold-preserved group, which was synchronized with early elevated NF-κB activity. It is well known that Kupffer cells were primed for the release of TNF-α during cold-preservation, and then activated at the time of reperfusion[26,28,29]. In addition, it has been previously reported that TNF-α producing activity of Kuffer cells increases with the duration of cold-ischemia in vitro[27]. That is, the presence of primed Kupffer cells during the extended cold preservation might result in overresponse to early NF-κB activity and excessive production of TNF-α in the initial phase of reperfusion.
In a rat model of warm hepatic ischemia, Jaeschke et al[27-29], observed a biphasic pattern of reperfusion injury. During the initial hour after reperfusion, Kupffer cells became activated and were the predominant source of oxygen free radicals. Up to 24 h thereafter, increased hepatic accumulation of neutrophils occurred in parallel to the progression of reperfusion injury[30]. In this study, neutrophils were sequestered earlier than warm ischemia, and increased significantly with prolonged cold-preservation. Neutrophil accumulation in the graft occurred within 0.5 h after reperfusion in the 24 h cold-preserved group, but early neutrophil accumulation was not synchronized with neutrophil activation, indicated by the high CD11b and low L-selectin expression. It was consistant with other studies that there was no rolling in sinusoids, and consequently selectin did not affect neutrophil accumulation in sinusoids[30], and furthermore, antibodies against β2-integrins had no effect on the initial neutrophil sequestration[31-33]. In addition, previous studies have shown that CD11b and L-selectin expressions were important to neutrophil-mediated tissue injury[20,34]. It implied that in a rat model of OLT, neutrophil-induced graft injury occurred within 2 h after reperfusion in the 18 h and 24 h groups, but graft injury at 0.5 h and 1 h was not neutrophil-dependent.
In summary, in the present study, we have demonstrated that NF-κB activation correlated with the expression of TNF-α, CINC, and ICAM-1 in vivo in the OLT model. Extended cold preservation might up-regulate TNF-α, CINC, and ICAM-1 expression in the graft, most probably through elevated NF-κB activation, and might contribute to neutrophil recruitment in sinusoids and subsequent neutrophil-mediated graft injury after liver transplantation. Thus, this chain reaction might play an important role in the development of early graft dysfunction after liver transplantation. We believe that elevated NF-κB activity is harmful to prolonged cold preservation, and inhibiting early NF-κB activity might protect against early graft injury after liver transplantation.