INTRODUCTION
CTLA4Ig is a soluble recombinant fusion protein constructed with an extracellular domain of mouse CTLA4 and Fc portion of human IgG. This protein binds to the mouse and rat B7-1/2 molecules, and blocks the co-stimulatory signals from antigen processing cell (APC) to antigen specific T cell. Treatment with CTLA4Ig gene transfection has been shown to prolong graft survival in mouse and rat heart, liver, pancreatic islet, kidney and lung transplantations and to induce donor-specific tolerance in some of these cases[1-7].
There are two methods of gene transduction: systemic administration (ie. intravenous injection) and local transfection of allograft. Gene transfer of sequences coding for soluble immunosuppressive molecules into transplanted organs aims to create a local microenvironment directly modulating the activation state of immune cells responsible for graft rejection[8]. Therefore, when compared with systemic administration, local and continuous production of biologically active compounds might increase their bioavailability and allow a more effective treatment. Furthermore, cells not involved in the rejection process could be spared, and side effects or generalized immunosuppression may thus be avoided.
Intragraft expression of CTLA4Ig by gene transfection at the time of transplantation can successfully prolong survival of several grafts[9-12]. Nevertheless, study of CTLA4Ig expression within the small bowel allograft has not been reported. In the present study, we transfected gene by ex vivo intra-superior mesenteric artery infusion of mCTLA4Ig cDNA packaged with lipofectin vector before transplantation, evaluated the local expression of CTLA4Ig gene and its effect on preventing acute rejection of small bowel allografts in rats.
MATERIALS AND METHODS
Animals and transplantation
Inbred male SD and Wister rats weighing 250 to 300 g were used as donors and recipients, respectively. All rats were obtained from the Animal Center of Nanjing Medical University.
After fasting for 24 h, donors and recipients were anesthetized with an intraperitoneal injection of pentobarbital (50 mg/kg). The vasculature of the donor small bowel was perfused with 10 mL heparinized saline solution at 4 °C. A segment of 20 cm small bowel with portal vein and superior mesenteric artery attached to a cuff of aorta were removed. The lumen of the donor small bowel perfused with 20 mL pure saline solution at 4 °C. The small bowel graft was transplanted with an end - to - side anastomosis of the cuff of aorta and portal vein of the graft to the infrarenal aorta and infrarenal vena cava (Figure 1). After revascularization of the graft, the oral end and the anal end of the small bowel graft were constructed as a stoma respectively through the right abdominal wall of the recipient. All animals had free access to water within 24 h after transplantation. Starting from postoperative d 1, they received standard rat chow.
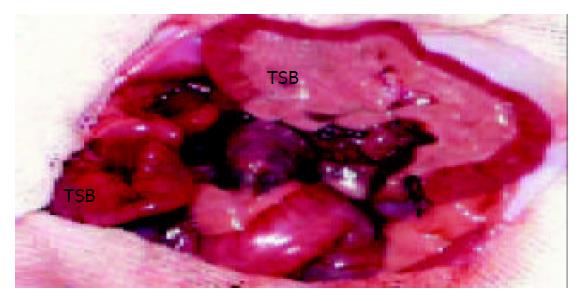
Figure 1 Heterotopic small bowel transplantation in the rat.
The vasculature of the graft has been anastomosed. RSB: recipient’s small bowel. TSB: transplanted small bowel.
Experimental groups
Animals were placed into two groups: One group of recipients did not receive treatment (control group, n = 21), and the other group of recipients received CTLA4Ig gene transfection (experimental group, n = 21).
Delivery of mCTLA4Ig to small intestine
The plasmid of AAVmCTLA4Ig was a kind gift of Professor I. Anegon (INSERM U437, Nants, France). DOTAP:Chole (in vivo GenSHUTTLE, Qbiogene) was used as the vector.
The AAVmCTLA4Ig was mixed with DOTAP:Chole at room temperature for 15 min to create the DNA-lipid complex. The final concentration of DNA in the complex was 0.5 μg/μL. Before cold preservation, the small bowel graft was irrigated with cold saline and then 50 μL lipid (control group) or 50 μL DNA-lipid complex (experimental group) was delivered into the superior mesenteric artery by slow infusion over 5-10 min. After 1.5 h of cold preservation, the superior mesenteric artery of small bowel was reperfused with 5 mL cold saline for 10 min before transplantation.
Graft histology, apoptosis detection and immunohistology
Three, seven and ten days after transplantation, 7 rats were killed in each group. Samples of the small bowel allografts were obtained. One half was fixed in paraformaldehyde for histology examination and cell apoptosis detection. Another half was preserved in nitrogen liquid for immunohistology.
For histology, sections from paraffin embedded blocks were stained with hematoxylin-eosin (H&E). Kuusanmaki’s protocol[13] served as basis for the grading of acute graft rejection and determination of diagnostic categories.
Apoptosis was detected on sections from paraffin-embedded blocks by the terminal deoxynucleotidyl transferase (TdTase) mediated d-UTP-biotin nick end labeling (TUNEL) technique[14-17]. Apoptosis assay with a detection kit from Bochinger (Mannheim, Germany), conformed to the manufacturer’s protocol strictly except that the sections were finally counterstained with methylgreen (Vector Laboratories). The nuclei of apoptotic cells were stained brown as detected under light microscope, and the number of apoptotic cells was determined by counting labeled enterocytes in 10 randomly chosen high-power fields[18].
Immunohistology was performed in cryostat sections. To detect CTLA4Ig in tissues, sections were subsequently incubated (60 min) with hamster anti-murine CTLA4 mAb (UC-4F10-11, BD Biosciences). Tissues probed with the mAb were then incubated with a biotin-conjugated mouse IgG-absorbed anti-hamster IgG Ab (60 min; G70-204 & G94-56, BD Biosciences), followed by HRP-conjugated streptavidin (45 min; Woburn MA) and DAB substrate, and sections were counterstained with hematoxylin.
Statistical analysis
Data were expressed as mean ± SD and Student’s t test was used. Significant difference was assumed when P < 0.05.
RESULTS
Immunohistology
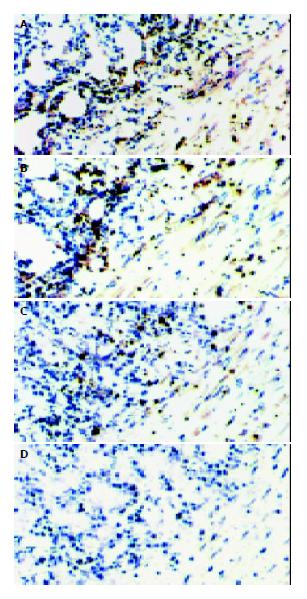
The CTLA4Ig expression of implanted small bowels was detected by immunohistology, and the small bowel grafts transduced with CTLA4Ig showed the presence of abundant labeling in mesenteric vascular walls, muscularis, submucosa and villus. Higher densities of CTLA4Ig were detected in grafts sampled at early time points, and persistent expression of CTLA4Ig was confirmed within 10 d after transplantation (Figure 2: A, B, C). As anticipated, there was no detectable expression of CTLA4Ig in the control small intestines (Figure 2: D).
Figure 2 Presence of CTLA4Ig in the small bowel allografts.
CTLA4Ig was stained as the brown granules. A, B, C represent the cryostat sections of CTLA4Ig gene transfected grafts on d 3, 7, 10 after transplantation, respectively. D represent the cryostat sections of non-CTLA4Ig gene transfected grafts, expression of CTLA4Ig was not detected. [original magnification ×200].
Morphological findings
In the control group, on d 3 after transplantation, only nonspecific changes were noted . Focal mesenteric inflammation, mild endothelial vacuolization, and minimal swelling and desquamation of enterocytes were observed in allografts, and a relatively normal villiform mucosal structure was retained. On d 7, a widespread inflammatory infiltrate in the mesentery, with moderate invasion of the intestinal wall, villous blunting and part of the crypt destruction, was noticed in allografts. In addition, endothelial swelling and proliferation with intimal thickening were found in small mesenteric arteries and arterioles. On d 10, a pronounced mesenteric infiltrate with severe invasion of the intestinal wall was found in allografts. Endothelial proliferation with intimal thickening resulted in luminal obliteration. Furthermore, moderate to extreme enterocystic necrosis could be recognized as erosions and focal ulcerations. However, the allografts treated with CTLA4Ig gene transfection showed nearly normal mucosal structure, slight cell infiltration and minimal swelling and desquamation of enterocytes on d 3, 7 and 10 post-transplantation.
Detection of apoptotic enterocytes by TUNEL
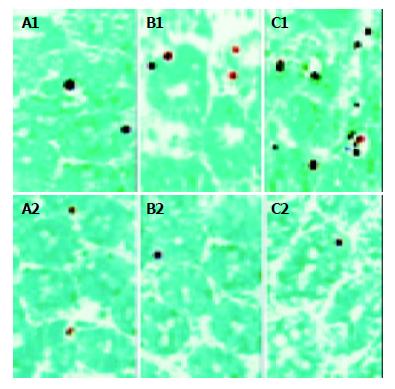
Apoptotic cells were detected mainly in the crypts. On d 3 after transplantation, a small number of labeled enterocytes were observed both in control (5.3 ± 1.5, n = 7) and experimental (5.8 ± 1.8, n = 7) groups. There was no significant difference in the number of apoptotic enterocytes between the two groups. On d 7, the number of labeled enterocytes increased dramatically (61.7 ± 2.8, n = 7) and reached a higher level on d 10 (101 ± 6.1, n = 7) in control group after transplantation (Figure 3: A1, B1, C1), whereas an increasing number of labeled nuclei could not be recognized in experimental group on d 7 (3.4 ± 1.0, n = 7) and on d 10 (3.6 ± 1.3, n = 7) after transplantation (Figure 3: A2, B2, C2). The difference in the number of apoptotic enterocytes in allografts between control and experimental groups on d 7 and 10 was extremely significant (bP < 0.0005, t = 41.2876 on day 7 and dP < 0.005, t = 39.2437 on d 10).
Figure 3 Apoptotic crypt cells in the small bowel allografts.
A1, B1, C1 represent the tissue sections of non-CTLA4Ig gene transfected grafts obtained on d 3, 7, 10 after transplantation, respectively. A2, B2, C2 represent the tissue sections of CTLA4Ig gene transfected grafts obtained on d 3, 7, 10 after transplantation, respectively. [original magnification ×400].
DISCUSSION
Small bowel transplantation has emerged as a life-saving therapy for patients with irreversible intestinal failure and is now a routine therapeutic tool at some transplant centers[19,20]. Nevertheless, graft rejection is still the major obstacle to the transplantation[21-23]. Blockade of the B7/CD28 co-stimulation signal by systemic transfer of CTLA4Ig gene through intravenous infusion of AdCTLA4Ig has been shown to prolong small intestinal graft survival[24]. However, different from heart, liver, kidney and other organs, the small bowel is unique among vascularized organ grafts with rich lymphoid components and large amounts of bacteria, severe infection as well as rejection occurs easier after transplantation. Local CTLA4Ig gene transfection of small bowel allograft could be the ideal therapeutic strategy of anti-rejection. It allows inhibition of the recipient’s rejection response, while maintaining the recipient’s efficient peripheral immune function (not suppressing the antibacterial response). The local transfection of CTLA4Ig gene has been successfully achieved in rat heart, liver, kidney, islet and lung allografts[9-12], but it has not been studied in the small bowel transplantation. In the present study, we perfused CTLA4Ig cDNA packaged with lipofectin vector via intra-superior mesenteric artery into the small bowels. By immunohistology, there were a large amount of CTLA4Ig expression in the small bowel allografts on days 3, 7 and 10 after transplantation. The result suggests that local CTLA4Ig gene transfection of small bowel allograft is feasible.
In non-CTLA4Ig gene transfected small bowel allografts, a histological change of progressive acute allograft rejection could be recognized. Although the allografts did not show any specific morphological changes on d 3, acute allograft rejection of grade I on d 7 and grade II on d 10 after transplantation was noticed. In contrast, no evidence of acute rejection was observed in CTLA4Ig gene transfected allografts on d 3, 7 and 10 after transplantation. These data indicate that local CTLA4Ig expression in small bowel allografts can prevent acute rejection after transplantation. The protection of local CTLA4Ig expression in small bowel allografts from acute rejection may be because of the inhibition of expansion of alloantigen-induced T cell priming. The local transfection of CTLA4Ig can block B7 ligand expression on the surface of intragraft APCs, and thus blocking B7/CD28 co-stimulatory pathway. In the absence of B7/CD28 co-stimulatory signal, the interaction of T cell with the alloantigen commonly produces T cell anergy[25-29].
Apoptosis is a DNA-dependent cell death mechanism, which occurs under physiological and pathological conditions. In organ transplantation, apoptosis is an important biochemical indicator of allograft rejection[14-17]. In the process of allograft rejection, the target cells damage is mainly mediated by cytotoxic T lymphocytes through the perforin-dependent granule-exocytosis pathway and the Fas/Fas ligand (FasL) pathway[30-32]. Both perforin- and Fas-mediated pathways of cytotoxicity can result in target cell apoptosis. Numerous reports have demonstrated up-regulation of transcripts for perforin, granzyme B, and FasL during allograft rejection[33-36]. In the present study, TUNEL illustrated a few apoptotic enterocytes on d 3 and showed a dramatically increased number of apoptotic enterocytes on d 7 and 10 after transplantation in non-CTLA4Ig gene transfected small bowel allografts. This result demonstrates the existence of acute rejection after transplantation. Nevertheless, in CTLA4Ig gene transfected small bowel allografts, a few apoptotic crypt cells was observed on days 3, but the number of apoptotic enterocytes did not increase on d 7 and 10 after transplantation. It indicates that the apoptosis in small bowel allografts can be prevented by local CTLA4Ig gene transfection, and the cytotoxic roles of infiltrated T lymphocytes and the acute rejection after transplantation can be prevented by CTLA4Ig expression in the allografts. The few apoptosis in allografts on d 3 was likely caused by cold preservasion damage and postischemic reperfusion damage[37-39].
In conclusion, local CTLA4Ig gene transfection of small bowel allograft can be achieved by perfusing CTLA4Ig cDNA packaged with lipofectin vector via intra-superior mesenteric artery into the small bowels, and the local CTLA4Ig expression in the allograft can prevent acute rejection after transplantation.
ACKNOWLEDGMENT
The authors are grateful to Professor I. Anegon (INSERM U437, Nantes, France) for the kind gift of the AAVmCTLA4IgG plasmid, and thank Professor Xirong Guo (Experimental Research Center of Nanjing Medical University) for the experimental help.