INTRODUCTION
Alpha-fetoprotein is the major serum protein in human embryos, and is synthesized by embryonic liver and yolk sac. During the embryonic growth course, AFP expresses much more (3 g/L), and falls to the adult level (0.02 × 10-3 g/L) after one-year birth. Although many investigations for the function of AFP had been carried out, the biological role of AFP is still a riddle so far. Because the composition and the sequence of the amino acid resides of AFP were very similar to those of human serum albumin, thus, people always think that the function of AFP just like human serum albumin, which functions to transport materials and stabilize blood colloid osmotic pressure in the life course of the fetus. However, the concentration of serum AFP increases apparently with liver cancer or liver optimum regeneration in humans. AFP always accompanies with the growth of liver cells, and it is possible that AFP may be related to the proliferation of tumor or fetal cells. Some investigations had showed that AFP could be individually synergy with other growth factors to promote the growth of many tumor or normal cells[1-6]. Alpha-fetoprotein (MW 69 ku) is a kind of biomacromolecules, and it is impossible to directly permeate the cells to regulate cell proliferation. We previously found that AFP could enhance growth of human hepatocellular carcinoma Bel 7402 and NIH3T3 cells, and there were two typical receptors of AFP existed on the membranes of these cells[7,8]. However, it has not been reported in former investigations how AFP influences the expression of oncogenes which are mediated by AFP receptors to regulate growth of human hepatocellular carcinoma cells. This study used human hepatocellular carcinoma Bel 7402 cell line, which is closely related to AFP, to observe mRNA expression of the oncogene c-fos, c-jun, and N-ras, and protein expression of mutative p53 and p21ras, which are correlated with cell proliferation, after treated with AFP. Additionally, the study explored some molecular mechanisms for AFP-mediated growth of human hepatocellular carcinoma cells.
MATERIALS AND METHODS
Materials
Human hepatocellular carcinoma Bel 7402 cells, crude AFP, and monoclonal anti-AFP antibody were provided by Endocrine Research Group of the Department of Biochemistry and Molecular Biology, Health Science Center, Peking University; RPMI 1640 medium was purchased from GIBCO; Fetal calf serum (FCS) was from the Blood Research Institute of Chinese Medicine Science Academy (Tianjin, China); Human serum albumin (HSA) and MOPS were purchased from Sigma Company; Diethyl pyrocarbonate (DEPC), sodium dodecyl sulfate (SDS), agarose, and Tris were obtained from Bio-Rad Company; Total RNA extraction kit was purchased from Promega Company; α-32P-dCTP was bought from YaHui Biology Engineer Company (Beijing, China); N-ras, c-fos, c-jun, and β-actin cDNA probes were from the Department of Endocrinology, Northwestern University (Chicago); Random primer labeling kit was the product of Takara company (Japan); Salmon sperm DNA, fraction V of bovine serum albumin (BSA), and Ficoll-400 were bought from the Jingke Chemical Reagents Company (Beijing, China); Monoclonal antibodies for mutative p53 and p21ras were from NEOMARKERS Company.
Methods
Purification of human AFP Human AFP was prepared by the method as described elsewhere[9]. Briefly, human cord blood AFP was precipitated by ammonium sulfate and passed through an anti-AFP antibody affinity chromatography column. The AFP-positive fractions were collected and concentrated. The purity of prepared AFP was 92.7% determined by SDS-polyacrylamide gel electrophoresis (SDS-PAGE). The protein was stored at -80 °C until analysis.
Cells culture Human hepatocellular carcinoma Bel 7402 cells (1.5 × 104/mL) were cultured in RPMI 1640 medium supplemented with 100 mL/L FCS at 37 °C in a humidified atmosphere of 50 mL/L CO2. The cultured medium was changed after 24 h.
RNA isolation and Northern blot analysis The cells were treated with AFP (20 mg/L), HSA (20 mg/L), anti-AFP antibody (40 mg/L), or AFP (20 mg/L) + anti-AFP antibody (40 mg/L) for 2, 6, 12, and 24 h, respectively. Total cellular RNA was isolated from Bel 7402 cells using a TRIzol reagent kit (Promega, Madison, WI) according to the manufacturer’s protocol. Then, RNA was quantitated by the absorbance at 260 nm, and RNA (10-20 μg/lane) was fractionated by electrophoresis through 10 g/L formaldehyde agarose gel. The fractionated RNA was transferred in 20 × SSC buffer to the nitrocellulose membrane (Millipore Corporation, Bedford, MA) using the standard procedure[10]. These membranes were hybridized with α-32P labeled probe, and washed using the standard protocol. The membranes were then exposed to the X-ray film at -80°C.
Purification of protein and Western blot analysis The cells were treated with AFP (20 mg/L), HSA (20 mg/L), anti-AFP antibody (40 mg/L), or AFP (20 mg/L) + anti-AFP antibody (40 mg/L) for 6, 12, and 24 h, respectively. After washed three times with PBS (pH 7.4, 0.15 mol/L), the cells were lyzed in 10 µL of lysis buffer containing 2 mL/L Triton X-100, 500 mmol/L NaCl, 500 mmol/L sucrose, 1 mmol/L EDTA, 0.15 mmol/L spermine, 0.5 mmol/L spermidine, 10 mmol/L HEPES (pH 8.0), 200 µmol/L phenylmethylsulfonyl fluoride, 2 mg /L leupeptin, 2 mg /L pepstatin, 24 000 IU /L aprotinin, and 7 mmol/L β-mercaptoethanol. Proteins (40 µg) were subjected to SDS-PAGE, and transferred to the PVDF membrane for immunodetection. The SDS-PAGE molecular weight markers (Bio-Rad) verified the correct location of the visualized bands. The membranes were blocked with 50 mL/L nonfat milk in PBS-Tween, probed with anti-p53 or anti-p21 antibody, and followed by secondary antibody (goat anti-mouse IgG-alkaline phosphatase). Immunoreactive proteins were detected using a color develop system (NBT/BCIP) by the standard procedure[10].
RESULTS
Expression of c-fos mRNA
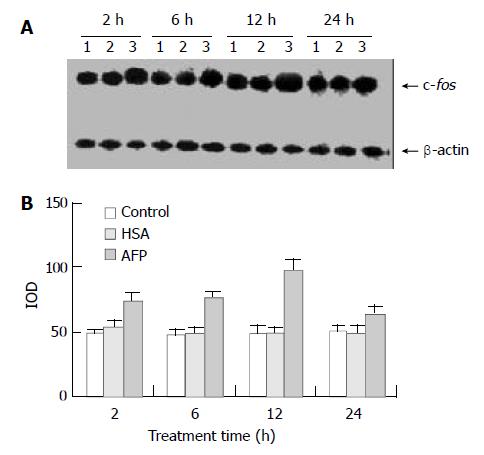
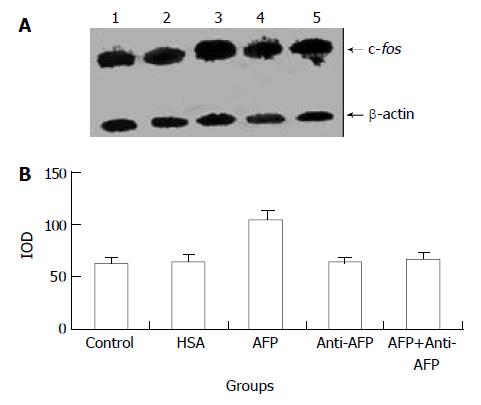
Northern blot analysis demonstrated the overexpression of c-fos mRNA in Bel 7402 cells after treated with AFP for 2, 6, and 12 h. The data showed that AFP (20 mg/L) treated for 2 h significantly increased the expression of c-fos mRNA in Bel 7402 cells by 51.1% compared with the control group. The expression of c-fos mRNA continuously increased by 60.9% and 96.0% when treated with AFP for 6 h and 12 h, respectively, and declined thereafter to 25.5% increase at 24 h, compared with control group (Figure 1). However, HSA at the same dosage (20 mg/L) as AFP and anti-AFP antibody (40 mg/L) had no significant influence on the expression of c-fos mRNA in Bel 7402 cells. Anti-AFP antibody partially blocked an increase in the expression of c-fos mRNA by AFP (Figure 2).
Figure 1 Effects of AFP (20 mg/L) or HSA (20 mg/L) on the expression of c-fos mRNA in human hepatocellular carcinoma Bel 7402 cells analyzed by Northern blot.
The cells were incu-bated with AFP or HSA for 2, 6, 12, and 24 h, respectively. A: Autoradiogram of Northern blot. Lane 1: control group; Lane 2: HSA treated group; Lane 3: AFP treated group. B: Densito-metric intensity of absorbance (IOD) of c-fos mRNA expres-sion in Bel 7402 cells. The data were selected from 3 indepen-dent experiments.
Figure 2 Effects of AFP (20 mg/L), HSA (20 mg/L), anti-AFP antibody (40 mg/L), and AFP (20 mg/L) + anti-AFP antibody (40 mg/L) on c-fos mRNA expression in human hepatocellular carcinoma Bel 7402 cells analyzed by Northern blot after 2 h treatment.
A: Autoradiogram of Northern blot. Lane 1: control group; Lane 2: HSA treated group; Lane 3: AFP treated group; Lane 4: anti-AFP antibody treated group; Lane 5: AFP + anti-AFP antibody treated group. B: Densitometric intensity of ab-sorbance (IOD) of c-fos mRNA expression in Bel 7402 cells. The data were selected from 3 independent experiments.
Expression of c-jun mRNA
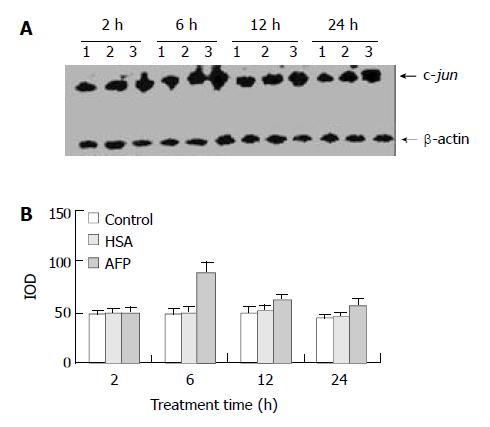
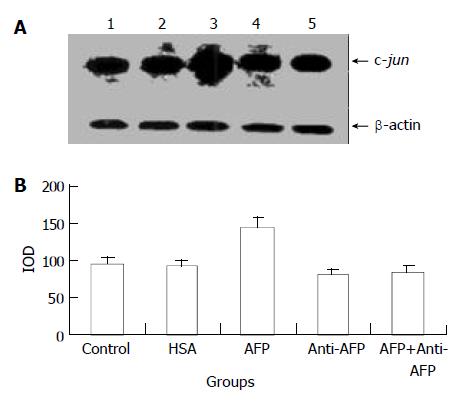
AFP (20 mg/L) had no significant influence on the expression of c-jun mRNA in human hepatocellular carcinoma cells when treated for 2 h, but when treated for 6 h the expression of c-jun mRNA increased obviously, the increase rate was 81.3%, but when treated for 24 h, increased rate fell to 14.6% as compared with the control group (Figure 3). However, HSA (20 mg/L) at the same dosage as AFP and anti-AFP antibody (40 mg/L) had no significant influence on the expression of c-jun mRNA in Bel 7402 cells. Anti-AFP antibody partially inhibited an increase in the expression of c-jun mRNA by AFP (Figure 4)
Figure 3 Effects of AFP (20 mg/L) or HSA (20 mg/L) on the expression of c-jun mRNA in human hepatocellular carcinoma Bel 7402 cells analyzed by Northern blot.
The cells were incu-bated with AFP or HSA for 2, 6, 12, and 24 h, respectively. A: Autoradiogram of Northern blot. Lane 1: control group; Lane 2: HSA treated group; Lane 3: AFP treated group. B: Densito-metric intensity of absorbance (IOD) of c-jun mRNA expres-sion in Bel 7402 cells. The data were selected from 3 similar experiments.
Figure 4 Effects of AFP (20 mg/L), HSA (20 mg/L), anti-AFP antibody (40 mg/L), and AFP (20 mg/L) + anti-AFP antibody (40 mg/L) on the expression of c-jun mRNA in human hepato-cellular carcinoma Bel 7402 cells analyzed by Northern blot after 6 h treatment.
A: Autoradiogram of Northern blot. Lane 1: control group; Lane 2: HSA treated group; Lane 3: AFP treated group; Lane 4: anti-AFP antibody treated group; Lane 5: AFP + anti-AFP antibody treated group. B: Densitometric intensity of absorbance (IOD) of c-jun mRNA expression in Bel 7402 cells. The data were selected from 3 independent experiments.
Expression of N-ras mRNA
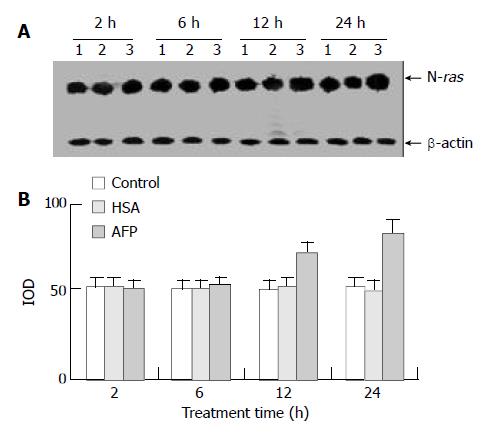
Northern blot analysis showed that AFP (20 mg/L) had no significant influence on the expression of N-ras mRNA in Bel 7402 cells when treated for 2 h and 6 h, but N-ras mRNA was overexpressed when treated with AFP (20 mg/L) for 12 h and 24 h. The increased ratios were 22.6% (12 h) and 59.9% (24 h), respectively, compared with the control group, and HSA (20 mg/L) did not significantly affect the expression of N-ras mRNA (Figure 5).
Figure 5 The effects of AFP (20 mg/L) or HSA (20 mg/L) on the expression N-ras mRNA in human hepatocellular carcinoma Bel 7402 cells analyzed by Northern blot.
The cells were incubated with AFP or HSA for 2, 6, 12, and 24 h, respectively. A: Autora-diogram of Northern blot. Lane 1: control group; Lane 2: HSA treated group; Lane 3: AFP treated group. B: Densitometric in-tensity of absorbance (IOD) of N-ras mRNA expression in Bel 7402 cells. The data were selected from 3 similar experiments.
Expression of p21ras protein
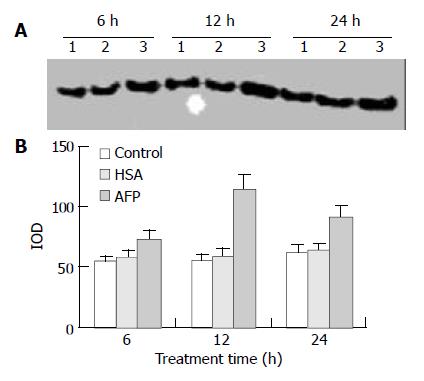
Western blot analysis demonstrated that AFP significantly enhanced the expression of p21ras protein in Bel 7402 cells by 35.2%, 102.6%, and 46.8% at 6, 12, and 24 h, respectively, compared with the control group. However, HSA (20 mg/L) did not influence the expression of p21ras protein (Figure 6).
Figure 6 Effects of AFP (20 mg/L) or HSA (20 mg/L) on the expression of p21ras in Bel 7402 cells analyzed by Western blot.
The cells were incubated with AFP or HSA for 6, 12, and 24 h, respectively. A: Western blot analysis. Lane 1: control group; Lane 2: HSA treated group; Lane 3: AFP treated group. B: Den-sitometric intensity of absorbance (IOD) of p21ras protein. The data were selected from 3 similar experiments.
Expression of mutative p53 protein
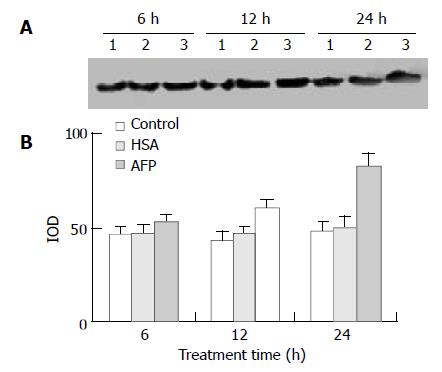
The data showed that AFP significantly influenced the expression of mutative p53 protein in Bel 7402 cells by 13.4%, 39.9%, and 70.6% at 6, 12, and 24 h, respectively, compared with the control group. However, HSA (20 mg/L) did not affect the expression of mutative p53 protein (Figure 7).
Figure 7 Effects of AFP (20 mg/L) or HSA (20 mg/L) on the expression of mutative p53 protein in Bel 7402 cells analyzed by Western blot.
The cells were treated with AFP or HSA for 6, 12, and 24 h, respectively. A: Western blot analysis. Lane 1: control group; Lane 2: HSA treated group; Lane 3: AFP treated group. B: Densitometric intensity of absorbance (IOD) of p53 protein. The data were selected from 3 similar experiments.
DISCUSSION
Many investigations have shown that AFP receptors exist on the membrane of various tumor cells[5,7,8,11-13], and play an important role in regulating growth of the cells[7,8,14]. The receptor may mediate intercellular signal transduction which influences the expression of genes related to proliferation, and the expression of these genes is the most direct factor that controls cell cycle. Our results showed that AFP stimulated the expression of c-fos, c-jun, and N-ras mRNA in hepatocellular carcinoma Bel 7402 cells. Both c-fos and c-jun have the characteristics of early response genes. When treated with AFP, the two oncogenes in Bel 7402 cells express promptly, thereafter the expression of these oncogenes dramatically declines. Previous researches had showed that AFP promoted the proliferation[1-6] and some oncogene expression of many tumor cells[15,16]. Cell growth was regulated by various factors, in which, some oncogene coding proteins have an important function in modulating growth and differentiation of the cells. c-fos and c-jun are the immediate early genes (IEG) with the characteristics of early response gene. When Bel 7402 cells were treated with AFP, the expression of these oncogenes had the characteristics of dynamics, which was independent on the translation of RNA. Additionally, IEG coding regulated-proteins can control the expression of some later response genes through the activation of nuclear transcription factors. Furthermore, c-Fos and c-Jun proteins can interact with each other to form a heterodimer (Fos-Jun) called AP-1 (activator protein-1). The dimer has leucine zipper structure, which has stronger binding affinity to DNA, and is the third messenger of signal transduction. The AP-1 protein is a nuclear transcription factor, which can regulate gene transcription by binding to a gene transcription-regulated element, and can increase transcriptional activation of downstream target genes. A previous study showed that the transcriptional properties of AP-1 depended on the site of cAMP-response elements to influence the expression of growth-related genes in hepatocellular carcinoma[17]. Therefore, we thought that AFP regulated cell cycle of Bel 7402 cells, which was related to the expression of early-response genes. Our result also showed that AFP promoted the expression of N-ras in Bel 7402 cells. The N-ras gene is closely related to cell proliferation, and p21ras, the N-ras coding protein, is an important intermediate of tyrosine-protein kinase (TPK) signal pathway in the cells to regulate growth-related gene expression. Therefore, we suggested that AFP could affect signal transduction to regulate growth of Bel 7402 cells probably through stimulating the expression of N-ras.
The wild type p53 is a very important tumor suppressor gene to inhibit proliferation of tumor cells. Some studies had showed that hepatocellular carcinoma was closely related to abnormal expression of p53 gene[18,19]. Additionally, p53 and beta-catenin mutation rates were inversely correlated in hepatocellular carcinoma[20]. The inactivation of p53 was an important cause of aberrant accumulation of beta-catenin, which was involved in both cell-cell interactions and wnt pathway-dependent cell fate determination in many cancer cells[20]. A previous study found that p53 mutation was correlated significantly with invasiveness including vascular permeation of hepatocellular carcinoma cells, and p53 mutation in the primary lesion was useful as an indicator of the biological behavior of recurrent hepatocellular carcinoma[21]. It showed that mutative p53 protein played a critical role in cell proliferation of hepatocellular carcinoma. The p53 gene could influence AFP expression to regulate the biological behavior of tumor cells[22,23]. Our data had showed that AFP could enhance the expression of mutative p53 protein. When p53 gene is mutated, it can not suppress the growth of tumor cells, on the contrary, it possesses the functions of oncogenes. There are two types of mutative p53 genes in tumor cells, one of the mutative p53 genes can restrain the growth-suppressing activity of wild type p53 gene, then display apparently its negative regulation; the other type of mutative p53 genes can cooperate with ras gene to actuate cell transformation, and then become a dominant oncogene, which promotes the growth of tumor cells. Because Ras protein is an important intermediate in TPK signal transduction pathway, we analyzed the expression of N-ras mRNA, and then further detected its protein in this study. The results showed that AFP promoted the expression of p21ras protein in Bel 7402 cells. The functions of p21ras protein are similar to those of guanylate binding protein, and it can phosphorylate the intermediate in the downstream of TPK signal pathway to activate mitogen-activated protein kinase (MAPK) and to participate in intercellular signal transduction. The p21ras protein can transduce the signal from TPK to threoine/serine protein kinase chain through Ras-Raf-MAPK signal pathway. These responses further activate some transcription factors which promote gene expression to enhance the growth of various cells. Our results demonstrated that AFP increased the expression of p21ras. Therefore, we speculated that AFP affected the expression of p53 and p21ras proteins through AFP receptor-mediated signal transduction pathway to accelerate the proliferation of Bel 7402 cells. Similar to the findings in this study, our previous study found that AFP enhanced the growth of HeLa and NIH3T3 cells through stimulating the expression of some oncogenes[8,15,16], suggesting that AFP might unselectively regulate proliferation of tumor cells in different tissues and species.
The mechanism for growth-promoting activity of AFP is still unclear. It has been known for a long time that AFP has the ability to transport the substances essential for cell proliferation to enter the cells. Studies have indicated that AFP is an important protein in the embryos to regulate growth of the fetus[24], and required for female fertility, but not essential for embryonic development[25]. Up to date, it is not clear whether the biological function of AFP is involved in regulating cell proliferation. Some studies indicated that AFP inhibited immune response to promote the growth of tumor cells in vivo[26,27]. Escaping from the surveillance of immune system was the primary cause for malignant growth of tumor cells. Hepatocellular carcinoma cells can escape from the surveillance of immune system through altering the expression of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) receptors[28], which can sustain the growth of tumor cells in vivo. However, our in vitro study could not establish the immune response without immune factors to affect the expression of the oncogenes in Bel 7402 cells. Therefore, it could not explain that AFP inhibited immune response to affect cell growth. Because AFP is a macromolecule, it is impossible to enter the cells through the cell membrane directly. Although AFP can transport some substances required for cell proliferation, it is not enough to completely support cell proliferation. Many studies found that AFP receptors existed on the membrane surface of various tumor cells, and mediated signal transduction to regulate the expression of the genes. The expression of these genes was the ultimate determination factor in regulating cell growth. Our results showed that AFP had the ability to stimulate the expression of the oncogenes c-fos, c-jun, and N-ras in Bel 7402 cells, and the response of these genes to AFP was various. Both the expression of c-fos and c-jun responded early, whereas the expression of N-ras and mutative p53 gene responded thereafter. Some oncogenes such as p53 and ras are important prognostic molecular markers in human hepatocellular carcinoma[29]. According to previous studies[5,7,8] and our results, we considered that the action of AFP was via TPK signal transduction pathway to stimulate the expression of some oncogenes which could regulate the growth of tumor cells. Many investigations had showed that the expression of some oncogenes was up-regulated in hepatocellular carcinoma, and some factors such as intergrin gene, p28/gankyrin, and HLA class I could influence cell proliferation of hepatocellular carcinoma[30-32]. However, some studies had shown that treatment of tumor cells in vitro with high dosage of AFP (1-10 μmol/L) significantly suppressed the growth of tumor cells, because AFP positively regulated cytochrome c-mediated caspase activation, apoptosome complex formation, and low-dose cytochrome c-mediated signals[33,34]. The mechanisms for how AFP regulates the expression of these genes and signal transduction, and why it is not essential for embryonic development[24], although it promotes the growth of some tumor cells[1-8] remain to be studied.
A previous study showed that AFP could coordinate other growth factors existed in HSA and serum to promote the growth of porcine granulosa cells, and AFP could function to modulate growth factor-mediated cell proliferation during development and neoplasia[4]. To further verify the up-regulation of these oncogenes was mediated by AFP, this study used HSA as a negative control due to similar amino acid sequences of AFP as HSA. The results showed that HSA or anti-AFP antibody did not stimulate the expression of these genes in Bel 7402 cells, but anti-AFP antibody efficiently blocked the function of AFP, which can be explained that AFP specifically stimulated the expression of these oncogenes in human hepatocellular carcinoma Bel 7402 cells.