Published online Mar 15, 2004. doi: 10.3748/wjg.v10.i6.800
Revised: September 2, 2003
Accepted: September 18, 2003
Published online: March 15, 2004
AIM: To evaluate the role of mitochondrial microsatellite instability (mtMSI) in gastric carcinogenesis.
METHODS: MtMSI was measured with PCR-single strand conformation polymophism (PCR-SSCP) in 68 cases of advanced gastric cancer, 40 cases of chronic gastritis, 30 cases of intestinal metaplasia and 20 cases of dysplasia.
RESULTS: MtMSI was observed in 12.5% (5 of 40) of chronic gastritis, 20.0% (6 of 30) of intestinal metaplasia, 25.0% (5 of 20) of dysplasia and 38.2% (26 of 68) of gastric cancer. These findings showed a sequential accumulation of mtMSI in the histological progression from chonic gastritis to gastric cancer. An association of mtMSI with intestinal histological type and distal location was found (P = 0.001 and P = 0.002), whereas no significant correlation was found between mtMSI and age at diagnosis, sex, tumor size, depth of invasion, lymph node spread and clinical stages (P > 0.05).
CONCLUSION: MtMSI may play an early and important role in the gastric carcinogenesis pathway, especially in the intestinal type and distal gastric cancer.
- Citation: Ling XL, Fang DC, Wang RQ, Yang SM, Fang L. Mitochondrial microsatellite instability in gastric cancer and its precancerous lesions. World J Gastroenterol 2004; 10(6): 800-803
- URL: https://www.wjgnet.com/1007-9327/full/v10/i6/800.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i6.800
The mechanisms of carcinogenesis in the gastric mucosa remain unclear. Genetic instability is strongly involved in n eo plastic tran sformation an d pro g ressio n [1-7]. In gastrointestinal carcinomas, such genetic instability may be classified into two different forms in which hypermutability occurs either due to chromosomal instability or due to microsatellite instability (MSI)[8-11]. MSI represents an important form of genomic instability associated with defective DNA mismatch repair in tumors. Although the MSI in nuclear DNA (nMSI) of gastric cancer has been established, little attention was paid to the MSI in mitochondrial DNA (mtMSI) in this cancer. In the present study, we analysed the mtMSI in gastric cancer and its premalignant lesions to elucidate whether mtMSI led to the progression from chronic gastritis to gastric cancer, via intestinal metaplasia and dysplasia.
Forty cases of chronic gastritis, 30 cases of intestinal mataplasia and 20 cases of dysplasia obtained from patients undergoing upper endoscopy for dyspepsia and 68 cases of surgically resected gastric cancer tissues were studied. Tissues from non-tumor or non-inflammatory gastric mucosa, showing no dysplasia or metaplasia, were used as a control in analysis of mtMSI. Hematoxylin-eosin (HE) staining was used for the histopathological diagnosis, evaluation and grading of gastritis, atrophy, intestinal metaplasia, dysplasia and cancer. Genomic DNA was isolated by standard proteinase-K digestion and phenol-chloroform extraction protocols. None of the patients with gastric cancer included in the present series had received chemotherapy or radiation therapy before operation.
PCR-single strand conformation polymorphism (PCR-SSCP) was performed to amplify the microsatellite sequence of mtDNA using published primers[18]. The primer consisted of 2 D-loop regions and 5 coding regions (Table 1). The reaction conditions and procedures were similar to those reported by Hebano et al[12].
| Repeatsequence | mtDNAregion | Position | Annealing(°C) | Primer (5’-3’) |
| (C)n | 270-425 | D-loop | 58 | TCCACACAGACATCAATAACA AAAGTGCATACCGCCAAAAG |
| (CA)n | 467-556 | D-loop | 55 | CCCATACTACTAATCTCATCAA TTTGGTTGGTTCGGGGTATG |
| (C)6 | 3 529-3 617 | ND1 | 55 | CCGACCTTAGCTCTCACCAT AATAGGAGGCCTAGGTTGAG |
| (A)7 | 4 555-4 644 | ND2 | 55 | CCTGAGTAGGCCTAGAAATAAA ACTTGATGGCAGCTTCTGTG |
| (T)7 | 9 431-9 526 | COIII | 55 | CCAAAAAGGCCTTCGATACG GCTAGGCTGGAGTGGTAAAA |
| (C)6 and (A)8 | 12 360-12 465 | ND5 | 55 | CACCCTAACCCTGACTTCC GGTGGATGCGACAATGGATT |
| (CCT)3 and (AGC)3 | 12 940-13 032 | ND5 | 55 | GCCCTTCTAAACGCTAATCC TCAGGGGTGGAGACCTAATT |
Each PCR was digested by appropriate restriction enzymes and electrophoresed at 300 V at 22 °C for 2 h on a 75g/L polyacrylamide gel containing 50 mmol/L boric acid, 1 mmol/L EDTA and 25g/L glycerol. After silver staining, PCR products that showed mobility shifts were directly sequenced using an appropriate internal primer and analyzed using the 373A automated DNA sequencer (Perkin Elmer Cetus). All analyses were repeated twice to rule out PCR artifacts.
Chi-square test with Yates’ correction was used. A P value < 0.05 was considered statistically significant.
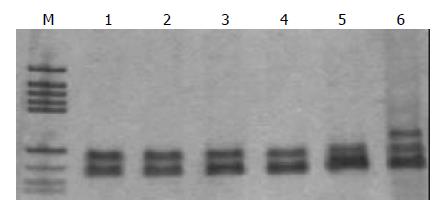
Sixty-eight gastric cancer samples and 90 benign gastric mucosal lesions were screened for mtMSI at seven repeat sites using the PCR-RFLP method. Figure 1 exhibits a representative mobility-shift band compared with normal counterpart. mtMSI was observed in 26 out of 68 cases (38.2%) of gastric cancer, 5 out of 40 cases (12.5%) of chronic gastritis, 6 out of 30 cases (20%) of intestinal metaplasia, and 5 out of 20 cases (25.0%) of dysplasia (Table 2).
| n | mtMSI(%) | |
| Chronic gastritis | 40 | 5(12.5) |
| Intestinal metaplasia | 30 | 6(20.0) |
| Dysplasia | 20 | 5(25.0) |
| Gastric cancer | 68 | 26(38.2) |
The clinicopathological characteristics of mtMSI-positive cases were compared with those of cases that were mtMSI-negative (Table 3). An association of mtMSI with intestinal histological type and distal location was found (P = 0.001 and P = 0.002), whereas no significant correlation was found between mtMSI and age at diagnosis, sex, tumor size, depth of invasion, lymph node spread and clinical stages (P > 0.05).
| Characteristic | n | mtMSI-positive | tMSI-negative |
| Age | |||
| <40 years | 15 | 5 | 10 |
| >40 years | 53 | 21 | 32 |
| Sex | |||
| Male | 42 | 15 | 27 |
| Female | 26 | 11 | 15 |
| Size | |||
| <5 cm | 38 | 14 | 24 |
| >5 cm | 30 | 12 | 18 |
| Histological type | |||
| Intestinal | 41 | 22b | 19 |
| Diffuse | 27 | 4 | 23 |
| Tumor location | |||
| Distal | 45 | 23c | 22 |
| Proximal | 23 | 3 | 20 |
| Invasion | |||
| Within the wall | 33 | 12 | 21 |
| Invading serosa | 35 | 14 | 21 |
| Lymph node spread | |||
| Absent | 30 | 11 | 19 |
| Present | 38 | 15 | 23 |
Carcinogenesis is a long-term, multistep process driven by multiple genetic and epigenetic changes in susceptible cells, which gain a selective growth advantage and undergo clonal expansion. Genetic instability is an important factor in the rapid accumulation of these genetic changes. Much attention has been directed to the genetic events in nDNA, such as activation of oncogenes, inactivation of tumor suppressor genes, and defects of mismatched DNA repair genes[13,14]. However, several aspects in the process of carcinogenesis are still unclear. It has been shown that somatic mutations in mtDNA were detected in various human tumors[15-18]. In addition, microsatellite instability has also been shown in mtDNA of colorectal and gastric carcinomas[18-20]. Further studies demonstrated that repeated mononucleotide alteration, missense mutation, and small deletion in NADH dehydrogenase genes and alteration in polycytidine (C)n tract in the D-loop region of mtDNA could occur in colorectal carcinomas[18]. These results imply that mtMSI of colorectal carcinomas may likely result from certain deficiencies in DNA repair. Therefore, it has been proposed that somatic mutations and mtMSI play a role in tumorigenesis and development of cancer[21,22]. To study the role of mtMSI in gastric carcinogenesis, we analyzed 68 cases of gastric cancer using seven microsatellite markers known to be altered in gastrointestinal carcinomas. MtMSI was found in 38.2% of patients with gastric cancer, implying that mtMSI may play an important role in the occurrence of a part of gastric cancers.
The majority of gastric carcinomas, particularly the “intestinal” type, which is most common in populations at high risk, were preceded by a precancerous stage, characterized by the following sequencial steps, namely chronic gastritis, intestinal metaplasia, and dysplasia[23,24]. Although numerous cytogenetic and molecular genetic studies have been performed on gastric adenocarcinomas, fundamental data pertaining to precursor lesions which could substantially clarify our understanding of the tumorigenesis in gastric mucosa are not available. This is the first study to examine the frequency of mtMSI in intestinal metaplasia and dysplasia, two premalignant lesions of gastric cancer in individuals without gastric cancer. If mtMSI plays an early and significant role in gastric carcinogenesis, one might expect to find mtMSI in metaplastic and dysplasia tissues before the development of cancer. In this study, mtMSI was detected in 12.5% of chronic gastritis, 20.0% of intestinal metaplasia, and 38.2% of gastric cancer tissues examined. These findings showed a sequential accumulation of mtMSI in the histological progression from chronic gastritis to cancer via intestinal metaplasia and dysplasia, suggesting an early and important role of mtMSI in the gastric carcinogenesis pathway, and they may define a subset of individuals susceptible to gastric cancer.
Cancers from different mutational pathways are thought to have different clinical features. nMSI+ gastric cancer was characterized by older age, antral location, intestinal type, lower prevalence of lymph node metastasis, and a lower pTNM stage[25,26]. However, the clinicopathologic characteristics of mtMSI+ gastric cancers remain unclear. In the current study, we did not find an obvious relationship between mtMSI and tumor size, depth of invasion, node metastasis or clinical stages, indicating a limited role of mtMSI in predicting the prognosis of gastric carcinomas. Gastric carcinomas can be divided into “intestinal” type and “diffuse ” type. A distinct genetic pathway has been found in gastric carcinogenesis of different histological subtypes and their tumor progression[27-29]. Increased beta-catenin mRNA levels and mutational alterations of APC and beta-catenin gene were present in intestinal type gastric cancer[30,31], whereas epigenetic inactivation of E-cadherin via promoter hypermethylation might be an early critical event in the development of undifferentiated tumors[32-35]. In this study, a marked difference in mtMSI was noted in gastric cancer. MtMSI was much more frequent in intestinal-type gastric cancers as compared with diffuse-type gastric cancer, suggesting that mtMSI is a predisposing event in intestinal type of gastric cancer. In contrast to mtMSI-negative gastric cancer, mtMSI-positive gastric tumors tended to exhibit a predominant distal location, similar to nMSI-positive gastric tumors.
The mechanisms underlying mtMSI in gastric mucosa remain unclear. In gastric mucosa, reactive oxygen species (ROS) are commonly released in inflamed gastric mucosa as a result of infection with Helicobacter pylori (H pylori), especially CagA+ strains, and they might be responsible for mtMSI-positive gastric cancer[36-38]. Mitochondrial genome was particularly susceptible to oxidative damage and mutation because of the high rate of ROS generation and inefficient DNA repair system in the organelle[39,40]. ROS and defective DNA repair were the two causes of increased damage proposed to explain mtMSI in H pylori -associated gastric cancer[41-43]. A possibly important role of H pylori in the development of mtMSI-positive gastric cancer needed to be further studied.
Although gastric cancer is a common disease, molecular markers for its early diagnosis are lacking. Mitochondrial DNA mutations occurred in a wide variety of cancers and might be usefull in the detection of cancer[44]. Indeed, some authors have implied that mitochondrial genome instability is so common and the enrichment of mutations in cancer is so significant that some mutations probably confer a selective or replicative advantage to those cells that have acquired such mutations[21]. Others have suggested that mtDNA mutations may enhance the toxicity of anti-cancer treatments[45-48]. Thus, the existence of mtDNA mutations in cancer may impact diagnosis and treatment and may be important in understanding the progression of some cancers. Given the early involvement of mtMSI in the multistep gastric carcinogenesis model, detection of mtMSI could serve as a surrogate marker for the risk of gastric cancer development[49]. It might help to identify high-risk patient, by determining mtMSI of preneoplastic lesions, such that close monitoring or potential intervention can be performed. Because the majority of patients with intestinal metaplasia and dysplasia will not progress to cancer and only a proportion of these patients harbore mtMSI, it is conceivable that patients with intestinal metaplasia and dysplasia displaying mtMSI are at greater risk of developing gastric cancer than those without mtMSI.
In conclusion, a high frequency of mtMSI can be found in gastric cancer and its premalignant lesions. Taking into consideration of the progressive increase in mtMSI frequency from premalignant to malignant lesions, our results suggest the early involvement and continuous accumulation of mtMSI in gastric cells that have entered the multistep gastric carcinogenesis pathway. The role of mtMSI in premalignant gastric lesions as a surrogate marker of the risk of gastric cancer development warrants further investigation.
| 1. | Lengauer C, Kinzler KW, Vogelstein B. Genetic instability in colorectal cancers. Nature. 1997;386:623-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1391] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 2. | Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260:816-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2062] [Cited by in RCA: 2066] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 3. | Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993;363:558-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1824] [Cited by in RCA: 1803] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 4. | Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4616] [Cited by in RCA: 4493] [Article Influence: 118.2] [Reference Citation Analysis (0)] |
| 5. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8087] [Cited by in RCA: 8096] [Article Influence: 224.9] [Reference Citation Analysis (1)] |
| 6. | Jen J, Kim H, Piantadosi S, Liu ZF, Levitt RC, Sistonen P, Kinzler KW, Vogelstein B, Hamilton SR. Allelic loss of chromosome 18q and prognosis in colorectal cancer. N Engl J Med. 1994;331:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 493] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 7. | White RL. Tumor suppressing pathways. Cell. 1998;92:591-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Fang DC, Jass JR, Wang DX, Zhou XD, Luo YH, Young J. Infrequent loss of heterozygosity of APC/MCC and DCC genes in gastric cancer showing DNA microsatellite instability. J Clin Pathol. 1999;52:504-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Fang DC, Yang SM, Zhou XD, Wang DX, Luo YH. Telomere erosion is independent of microsatellite instability but related to loss of heterozygosity in gastric cancer. World J Gastroenterol. 2001;7:522-526. [PubMed] |
| 10. | Martins C, Kedda MA, Kew MC. Characterization of six tumor suppressor genes and microsatellite instability in hepatocellular carcinoma in southern African blacks. World J Gastroenterol. 1999;5:470-476. [PubMed] |
| 11. | Wu BP, Zhang YL, Zhou DY, Gao CF, Lai ZS. Microsatellite instability, MMR gene expression and proliferation kinetics in colorectal cancer with famillial predisposition. World J Gastroenterol. 2000;6:902-905. [PubMed] |
| 12. | Habano W, Nakamura S, Sugai T. Microsatellite instability in the mitochondrial DNA of colorectal carcinomas: evidence for mismatch repair systems in mitochondrial genome. Oncogene. 1998;17:1931-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 124] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Fang DC, Wang RQ, Yang SM, Yang JM, Liu HF, Peng GY, Xiao TL, Luo YH. Mutation and methylation of hMLH1 in gastric carcinomas with microsatellite instability. World J Gastroenterol. 2003;9:655-659. [PubMed] |
| 14. | Fang DC, Luo YH, Yang SM, Li XA, Ling XL, Fang L. Mutation analysis of APC gene in gastric cancer with microsatellite instability. World J Gastroenterol. 2002;8:787-791. [PubMed] |
| 15. | Fliss MS, Usadel H, Caballero OL, Wu L, Buta MR, Eleff SM, Jen J, Sidransky D. Facile detection of mitochondrial DNA mutations in tumors and bodily fluids. Science. 2000;287:2017-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 612] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 16. | Richard SM, Bailliet G, Páez GL, Bianchi MS, Peltomäki P, Bianchi NO. Nuclear and mitochondrial genome instability in human breast cancer. Cancer Res. 2000;60:4231-4237. [PubMed] |
| 17. | Yeh JJ, Lunetta KL, van Orsouw NJ, Moore FD, Mutter GL, Vijg J, Dahia PL, Eng C. Somatic mitochondrial DNA (mtDNA) mutations in papillary thyroid carcinomas and differential mtDNA sequence variants in cases with thyroid tumours. Oncogene. 2000;19:2060-2066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 137] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Habano W, Sugai T, Yoshida T, Nakamura S. Mitochondrial gene mutation, but not large-scale deletion, is a feature of colorectal carcinomas with mitochondrial microsatellite instability. Int J Cancer. 1999;83:625-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Habano W, Sugai T, Nakamura SI, Uesugi N, Yoshida T, Sasou S. Microsatellite instability and mutation of mitochondrial and nuclear DNA in gastric carcinoma. Gastroenterology. 2000;118:835-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Máximo V, Soares P, Seruca R, Rocha AS, Castro P, Sobrinho-Simões M. Microsatellite instability, mitochondrial DNA large deletions, and mitochondrial DNA mutations in gastric carcinoma. Genes Chromosomes Cancer. 2001;32:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Hochhauser D. Relevance of mitochondrial DNA in cancer. Lancet. 2000;356:181-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Liu MR, Pan KF, Li ZF, Wang Y, Deng DJ, Zhang L, Lu YY. Rapid screening mitochondrial DNA mutation by using denaturing high-performance liquid chromatography. World J Gastroenterol. 2002;8:426-430. [PubMed] |
| 23. | Correa P, Shiao YH. Phenotypic and genotypic events in gastric carcinogenesis. Cancer Res. 1994;54:1941s-1943s. [PubMed] |
| 24. | Leung WK, Kim JJ, Kim JG, Graham DY, Sepulveda AR. Microsatellite instability in gastric intestinal metaplasia in patients with and without gastric cancer. Am J Pathol. 2000;156:537-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 121] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Wu MS, Lee CW, Shun CT, Wang HP, Lee WJ, Chang MC, Sheu JC, Lin JT. Distinct clinicopathologic and genetic profiles in sporadic gastric cancer with different mutator phenotypes. Genes Chromosomes Cancer. 2000;27:403-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 26. | Wu MS, Chang MC, Huang SP, Tseng CC, Sheu JC, Lin YW, Shun CT, Lin MT, Lin JT. Correlation of histologic subtypes and replication error phenotype with comparative genomic hybridization in gastric cancer. Genes Chromosomes Cancer. 2001;30:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 27. | Wu MS, Shun CT, Wang HP, Sheu JC, Lee WJ, Wang TH, Lin JT. Genetic alterations in gastric cancer: relation to histological subtypes, tumor stage, and Helicobacter pylori infection. Gastroenterology. 1997;112:1457-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Endoh Y, Sakata K, Tamura G, Ohmura K, Ajioka Y, Watanabe H, Motoyama T. Cellular phenotypes of differentiated-type adenocarcinomas and precancerous lesions of the stomach are dependent on the genetic pathways. J Pathol. 2000;191:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 29. | Ohmura K, Tamura G, Endoh Y, Sakata K, Takahashi T, Motoyama T. Microsatellite alterations in differentiated-type adenocarcinomas and precancerous lesions of the stomach with special reference to cellular phenotype. Hum Pathol. 2000;31:1031-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Ebert MP, Fei G, Kahmann S, Müller O, Yu J, Sung JJ, Malfertheiner P. Increased beta-catenin mRNA levels and mutational alterations of the APC and beta-catenin gene are present in intestinal-type gastric cancer. Carcinogenesis. 2002;23:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 118] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Park WS, Oh RR, Park JY, Lee SH, Shin MS, Kim YS, Kim SY, Lee HK, Kim PJ, Oh ST. Frequent somatic mutations of the beta-catenin gene in intestinal-type gastric cancer. Cancer Res. 1999;59:4257-4260. [PubMed] |
| 32. | Tamura G, Sato K, Akiyama S, Tsuchiya T, Endoh Y, Usuba O, Kimura W, Nishizuka S, Motoyama T. Molecular characterization of undifferentiated-type gastric carcinoma. Lab Invest. 2001;81:593-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Becker KF, Atkinson MJ, Reich U, Becker I, Nekarda H, Siewert JR, Höfler H. E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res. 1994;54:3845-3852. [PubMed] |
| 34. | Ascaño JJ, Frierson H, Moskaluk CA, Harper JC, Roviello F, Jackson CE, El-Rifai W, Vindigni C, Tosi P, Powell SM. Inactivation of the E-cadherin gene in sporadic diffuse-type gastric cancer. Mod Pathol. 2001;14:942-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Machado JC, Oliveira C, Carvalho R, Soares P, Berx G, Caldas C, Seruca R, Carneiro F, Sobrinho-Simöes M. E-cadherin gene (CDH1) promoter methylation as the second hit in sporadic diffuse gastric carcinoma. Oncogene. 2001;20:1525-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 196] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 36. | Wu AH, Crabtree JE, Bernstein L, Hawtin P, Cockburn M, Tseng CC, Forman D. Role of Helicobacter pylori CagA+ strains and risk of adenocarcinoma of the stomach and esophagus. Int J Cancer. 2003;103:815-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 37. | Farinati F, Cardin R, Degan P, Rugge M, Mario FD, Bonvicini P, Naccarato R. Oxidative DNA damage accumulation in gastric carcinogenesis. Gut. 1998;42:351-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 192] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 38. | Drake IM, Mapstone NP, Schorah CJ, White KL, Chalmers DM, Dixon MF, Axon AT. Reactive oxygen species activity and lipid peroxidation in Helicobacter pylori associated gastritis: relation to gastric mucosal ascorbic acid concentrations and effect of H pylori eradication. Gut. 1998;42:768-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 94] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci USA. 1997;94:514-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1282] [Cited by in RCA: 1381] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 40. | Li JM, Cai Q, Zhou H, Xiao GX. Effects of hydrogen peroxide on mitochondrial gene expression of intestinal epithelial cells. World J Gastroenterol. 2002;8:1117-1122. [PubMed] |
| 41. | Bagchi D, McGinn TR, Ye X, Bagchi M, Krohn RL, Chatterjee A, Stohs SJ. Helicobacter pylori-induced oxidative stress and DNA damage in a primary culture of human gastric mucosal cells. Dig Dis Sci. 2002;47:1405-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Shimoyama T, Fukuda S, Liu Q, Nakaji S, Fukuda Y, Sugawara K. Production of chemokines and reactive oxygen species by human neutrophils stimulated by Helicobacter pylori. Helicobacter. 2002;7:170-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Bohr VA, Stevnsner T, de Souza-Pinto NC. Mitochondrial DNA repair of oxidative damage in mammalian cells. Gene. 2002;286:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 150] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 44. | Bianchi NO, Bianchi MS, Richard SM. Mitochondrial genome instability in human cancers. Mutat Res. 2001;488:9-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 132] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 45. | Shen ZY, Shen J, Li QS, Chen CY, Chen JY, Yi Z. Morphological and functional changes of mitochondria in apoptotic esophageal carcinoma cells induced by arsenic trioxide. World J Gastroenterol. 2002;8:31-35. [PubMed] |
| 46. | Peters U, Preisler-Adams S, Lanvers-Kaminsky C, Jürgens H, Lamprecht-Dinnesen A. Sequence variations of mitochondrial DNA and individual sensitivity to the ototoxic effect of cisplatin. Anticancer Res. 2003;23:1249-1255. [PubMed] |
| 47. | Carew JS, Zhou Y, Albitar M, Carew JD, Keating MJ, Huang P. Mitochondrial DNA mutations in primary leukemia cells after chemotherapy: clinical significance and therapeutic implications. Leukemia. 2003;17:1437-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 128] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 48. | Wardell TM, Ferguson E, Chinnery PF, Borthwick GM, Taylor RW, Jackson G, Craft A, Lightowlers RN, Howell N, Turnbull DM. Changes in the human mitochondrial genome after treatment of malignant disease. Mutat Res. 2003;525:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 49. | Hiyama T, Tanaka S, Shima H, Kose K, Kitadai Y, Ito M, Sumii M, Yoshihara M, Shimamoto F, Haruma K. Somatic mutation of mitochondrial DNA in Helicobacter pylori-associated chronic gastritis in patients with and without gastric cancer. Int J Mol Med. 2003;12:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
Edited by Zhang JZ and Wang XL Proofread by Xu FM