Published online Feb 15, 2004. doi: 10.3748/wjg.v10.i4.586
Revised: September 12, 2003
Accepted: October 20, 2003
Published online: February 15, 2004
AIM: Anaemia caused by acute upper gastrointestinal bleeding is treated with blood transfusion or iron, but patients usually face a two-month recovery period from post-haemorrhage anaemia. This prospective, randomised, open, pilot study was designed to investigate whether recombinant human erythropoietin (Epoetin) therapy accelerate haematocrit increase in the post-bleeding recovery period.
METHODS: We studied hospitalised patients admitted because of acute ulcer bleeding or haemorrhagic gastritis, who had a haematocrit of 27%-33% and did not receive blood transfusions. One day after the endoscopic confirmation of cessation of bleeding, they were randomised either to erythropoietin (20000 IU Epoetin alfa subcutaneously, on days 0, 4 and 6) plus iron (100 mg im, on days 1 - 6, (G1) or iron only (G2). Haematocrit was measured on days 0, 6, 14, 30, 45, and 60, respectively.
RESULTS: One patient from G1 and two from G2 were lost to follow-up. Therefore, 14 and 13 patients from G1 and G2 respectively were analysed. Demographic characteristics, serum iron, ferritin, total iron binding capacity, reticulocytes, and haematocrit were not significantly different at entry to the study. Median reticulocyte counts were significantly different between groups on day six (G1: 4.0, 3.0-6.4 vs G2: 3.5, 2.1%-4.4%, P = 0.03) and median haematocrit on day fourteen [G1: 35.9, 30.7-41.0 vs G2: 32.5, 29.5%-37.0% (median, range), P = 0.04].
CONCLUSION: Erythropoietin administration significantly accelerates correction of anemia after acute ulcer bleeding. The haematocrit gain is equivalent to one unit of transfused blood two weeks after the bleeding episode.
- Citation: Ladas SD, Polymeros D, Pagonis T, Triantafyllou K, Paspatis G, Hatziargiriou M, Raptis SA. Does recombinant human erythropoietin accelerate correction of post-ulcer-bleeding anaemia A pilot study. World J Gastroenterol 2004; 10(4): 586-589
- URL: https://www.wjgnet.com/1007-9327/full/v10/i4/586.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i4.586
Following an episode of acute gastrointestinal bleeding from peptic ulcer disease, patients face a two-month recovery period from post haemorrhage iron deficiency anaemia. If haematocrit is lower than 25%, corresponding to a haemoglobin value of 8 g/dl, patients are usually treated with blood transfusion supplemented with iron replenishment therapy. However, when haematocrit is higher than 30%, it slowly recovers with iron administration alone. During this recovery period patients are unable to work and face health-related reduced quality of life, because of iron deficiency anaemia, at least until haematocrit is increased to more than 35%. Therefore, shortening the recovery phase from post-haemorrhage anaemia is of clinical importance.
Erythropoietin is a glycoprotein hormone, synthesized predominantly in the kidney, that promotes the differentiation of erythroid progenitor cells and therefore it regulates the production of red blood cells. In addition, it stimulates the synthesis of haemoglobin[1]. In the recent years, recombinant human erythropoietin (r-HuEPO, Epoetin Alfa) has become readily available and permitted the clinical investigation and application of this hormone to the treatment of anaemia. Epoetin Alfa has been shown to accelerate erythropoiesis and reduce the requirement for blood transfusion in patients undergoing gynaecologic surgery[2] and those with anaemia because of chronic renal failure[3], inflammatory bowel disease[4], or human immunodeficiency virus infection[5]. The success of Epoetin Alfa in treating anaemia in the post surgical recovery period[6] suggests that it may be of benefit as an adjuvant therapy in treating the post haemorrhage anaemia due to peptic ulcer bleeding. The aim therefore of the present pilot study was to investigate whether adjuvant therapy with erythropoietin could be of benefit to this patient population, by accelerating an increase of the haematocrit during the recovery period from anaemia.
Over a two-year period (1998-1999), 30 hospitalised adult patients admitted to our departments because of acute gastrointestinal bleeding from peptic ulcer or haemorrhagic gastritis were invited to participate in this prospective, randomised open pilot study. Patients included in the study fulfilled the following criteria. They did not require blood transfusion for resuscitation. They also had a stable haematocrit value at between 30% - 33% for men (normal range: 39% - 49%) and 25% - 30% for women (normal range: 33% - 43%) after confirming the cessation of the bleeding episode by endoscopy performed on the third day of hospitalisation. All patients should have a clean-based ulcer or red spot, indicating a low (< 3%) rebreeding rate[7]. Exclusion criteria were any known causes of anaemia including haematologic diseases, cancer, gastric surgery, or intention to use non-steroidal anti-inflammatory drugs following hospital discharge. Women of childbearing age, those with uncontrolled hypertension or seizure history and patients with severe chronic diseases were also excluded from the study.
Patients gave written informed consent to the study, after full explanation by the investigators. The study protocol was approved by our institution review board on human studies.
Study design Patients eligible to enter the study had haematocrit determinations on the second and third day of hospitalisation. If haematocrit had not changed by more than 1%, they were randomised according to a computer-generated randomisation list to Epoetin plus iron or iron therapy alone. Neither patients nor investigators were blinded to the therapy.
All patients were treated with intramuscular injections of 332 mg/2 ml ferric hydroxide polymaltose complex, corresponding to 100 mg of Fe3+, (Ferrum Hausmann®, Vifor, St. Gallen, Switzerland). Injections were given on days one to six. The total quantity of injected Fe3+ was 600 mg. Those randomised into the Epoetin group received adjuvant therapy with 20000 IU of recombinant human erythropoietin (Epoetin Alfa, Eprex®, Cilag, Switzerland) given subcutaneously on days zero, four and six. The total quantity of Epoetin given was 60000 IU. Patients were also treated with 20 mg of omeprazole twice daily, iv, for peptic ulcer bleeding over the initial 3-5 days of hospitalisation. They then resumed a normal diet and received 20 mg of omeprazole/day, orally, for one month. In those patients who were H pylori positive, eradication therapy was scheduled after completing follow-up, i.e., two months after the bleeding episode.
Haematocrit and reticulocytes (normal value: 0.5% - 1.5%) were measured on days 0, 6, 14, 30, 45 and 60, respectively. Serum iron (normal values: 50-160 μg/dl), ferritin (normal values: 12-300 μg/dl) and total iron binding capacity (TIBC) (normal values: 250-400 μg/dl) were measured on days 0, 14, 30 and 45 from randomisation.
Data were analysed by the statistical packages SPSS 10.0.0 (SPSS Inc., Chicago, Illinois, USA) and Statgraphics Plus 4.0 (Manugistics Inc., Statistical Graphics Corp., Rockville, USA). Numerical data were assessed by the non-parametric Mann-Whitney two-sided U-test and the Kruskal-Wallis t test used for two- and multiple-sample comparison analysis, respectively.
Results in the text and table are presented as median with ranges. Data in figures are presented as box-and-whisker plots. The box includes 50% of the results falling between 25th and 75th percentile (interquartile distance). The median value is represented as a horizontal line inside the box. Outliers, i.e., points more than 1.5 times the interquartile range from the end of the box are shown as open circles. A P value less than 0.05 was considered statistically significant.
Over the two-year study period, 30 adults fulfilling the inclusion criteria were randomised into the two treatment arms. Demographic and laboratory characteristics were not significantly different between the two groups at entry to the study (Table 1). Three patients were lost to follow up. Therefore, 14 patients in the Epoetin-iron treatment group and 13 patients in the iron treatment group were available for analysis.
| Epoetin+Iron (G1, n = 15) | Iron (G2, n = 15) | aP-value | |
| Male/female | 10/5 | 10/5 | |
| Age (years) | 62, 36-70 | 58, 35-71 | 0.54 |
| Ulcer/haemorrhagic gastritis | 13/2 | 12/3 | |
| Lost to follow up | 1 | 2 | |
| Haematocrit (%)b | 29.0, 26.0-33.0 | 28.8, 26.0-32.5 | 0.9 |
| Reticulocytes (%)b | 3.3, 2.4-3.9 | 2.8, 2.3-4.2 | 0.63 |
| Serum iron (μg/dl)b | 37, 18-54 | 38, 24-63 | 0.37 |
| Ferritin (μg/dl)b | 78, 20-147 | 64, 41-173 | 0.96 |
| TIBC (μg/dl)b | 302, 224-392 | 302, 250-391 | 0.92 |
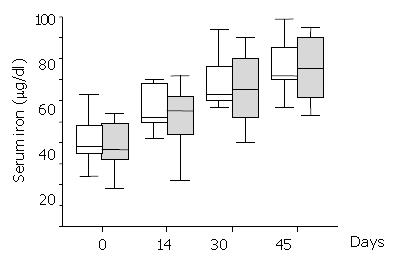
Serum iron levels significantly increased over the study period within each group (Figure 1), but they were not significantly different between treatment groups anytime during the study period (Mann-Whitney U test > 62, P > 0.67). All the patients had normal serum ferritin levels during the study period. Ferritin did not significantly change over time either in the Epoetin-iron treatment group (Kruskal-Wallis T = 0.07, P = 0.1) or the iron treatment group (Kruskal-Wallis T = 7.47, P = 0.06). There was not any significant difference of ferritin levels between treatment groups anytime during the study (Mann-Whitney U test > 46.5, P > 0.14). Similar results to ferritin were obtained for TIBC, which did not significantly change over time either within the Epoetin-iron (Kruskal-Wallis T = 2.87, P = 0.41) or the iron treatment group (Kruskal-Wallis T = 3.50, P = 0.32). In addition, there was not any significant difference of TIBC between treatment groups anytime during the study (Mann-Whitney U test > 58.5, P > 0.22).
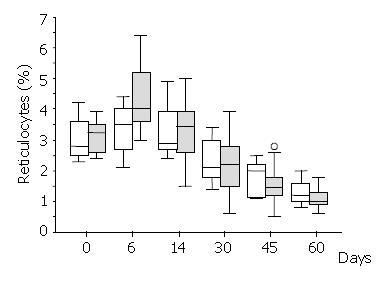
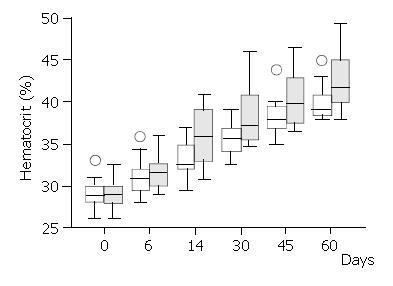
There was a brisk increase of reticulocyte counts in both groups on the sixth day of the study. This was the only date when there was a significant difference between the treatment groups in favour of the Epoetin-iron group (median: 4.0% versus 3.5%,) (Mann-Whitney U test = 46.5, P < 0.03) (Figure 2). Following the sixth day of the study, the reticulocyte counts were progressively decreased in both groups. Haematocrit increased over the study period within each group (Figure 3), but it was significantly different between treatment groups (higher in the Epoetin-iron group) only on the fourteenth day of the study (median: 35.85% versus 32.5%, range: 30, 7-41 versus 29, 5-37) (Mann-Whitney U test = 49, P = 0.041) (Figure 3). Epoetin therapy was not associated with any side effects in our study.
Acute upper gastrointestinal bleeding is a major cause of hospitalisation. Data from the USA and UK indicate that the overall incidence of acute upper gastrointestinal bleeding was about 100 hospital admissions per 100000 adult population per year[8,9]. In about 50% of these patients, the causes of bleeding were peptic ulcer or erosive-haemorrhagic gastritis. There are no guidelines dictating when patients with acute peptic ulcer bleeding should undergo transfusion. However, common sense dictates that stable patients, i.e., those who did not continue to bleed and had a low risk of rebleeding[10], as confirmed by emergency endoscopy, or a haematocrit higher than 25% should avoid blood transfusion[11]. Hui and co-authors have recently shown that patients under the age of 60 admitted because of bleeding duodenal ulcer, having no stigmata of haemorrhage on emergency endoscopy and no concurrent serious medical illness might be discharged within 24 hours of endoscopy if haematocrit was above 30% and there were no clinical signs of circulatory instability[12]. One might therefore, argue that our patients were candidates for early discharge. However, the prolongation of hospitalization in our study was only due to study practicalities, since the Epoetin-iron therapy could be safely administered on an outpatient basis.
Avoiding blood transfusion is wise in this group of patients. There is a worldwide concern of blood born transmission of infectious agents, and the risk of significant reduction in the blood donor pool with the planned introduction of testing for prior disease. The risk of infection per blood unit transfused was estimated by mathematical models to 1:676000 for HIV, 1:103000 for HCV and 1:63000 for HBV infection[13,14]. Transmission of hepatitis A or G virus was rare[14] and occurred primarily in the window period[15]. The most common infectious agents transmitted were cytomegalovirus[16] and Yersinia enterocolitica[17]. The immunologic risks ranged from mild febrile nonhaemolytic reactions to fatal haemolytic ones[18]. In addition, transfusion-associated graft-versus-host disease might be seen in immunocompetent patients[18,13].
Hospitalised patients for acute upper gastrointestinal bleeding may avoid blood transfusions, but following their discharge from the hospital they face a two-month recovery period from acute post haemorrhage anaemia. Symptoms experienced by anaemic patients included cold skin, dizziness, palpitations, or depression, which substantially affect patients’ quality of life. Identifying patients most likely to benefit from blood conservation is not always easy. There was a recent debate about the threshold for transfusion at which the benefits outweigh the risks[18]. No doubt even elderly patients might tolerate haematocrit of about 20% - 22%, especially those with megaloblastic anaemia. However, patients having haematocrit of about 30% at discharge might feel unable to work until haematocrit recovers to about 35%. It is therefore of clinical importance to improve the functional status of the anaemic patients by shortening the recovery period from iron deficiency anaemia.
Recombinant human erythropoietin (Epoetin Alfa) has been shown to accelerate erythropoiesis in certain anaemia states[3-5,18]. It has also been used perioperatively in gynaecologic surgery[2,6] and in a few cases of Jehovah’s Witness hospitalised for acute gastrointestinal bleeding[20-22,25]. Epoetin Alfa may therefore allow a lower value of haematocrit to be accepted, above which one would not transfuse. Our data agree with published studies showing an increase of haematocrit in the erythropoietin treated anaemic patients between one and two weeks post treatment[2,6,23,24]. They also showed that patients with post haemorrhage anaemia treated with erythropoietin plus iron had a higher median haematocrit (35.9%) two weeks after the bleeding episode compared with controls treated with iron only (32.5%). This gain of 3.4% of haematocrit corresponds to one unit of transfused blood.
We used an adequate iron dose to replenish iron stores, as it was shown by normal ferritin and serum iron levels in all patients throughout the two-month study period. This is essential for patients treated with recombinant human erythropoietin, because it stimulates erythropoiesis and may quickly exhaust iron stores. We selected the intramuscular iron formulation to avoid dark-stained stools that might be mistaken for recurrence of bleeding. However, since oral iron is preferred to intramuscular injections, further studies are needed to investigate whether oral iron therapy may have the same beneficial result simplifying the therapeutic regime of iron-Epoetin, and avoiding possible side effects of iron injections.
Various doses of Epoetin have been used to stimulate erythropoiesis ranging from 50 to 600 IU/kg. In the study of Rutherford et al[24] higher doses of Epoetin were associated with quicker stimulation of erythropoiesis, indicated by a significant increase of haematocrit by the sixth day. Our patients were treated with a lower dose of Epoetin (300 IU/kg), which may explain why they had a later response (day 14).
Our pilot study has demonstrated that patients who have been treated with Epoetin Alfa in addition to iron have accelerated response in terms of erythropoiesis. However, Epoetin is an expensive drug, the cost per treatment was 2.5 Euro for iron and 533.5 Euro for the Epoetin regime in our study. A larger study with a different design should address the secondary end-points of symptom scores and quality of life changes and also the socio-economic consequences like the time lost from work and the time of resumption of activities. The usefulness of Epoetin as a blood conserving adjuvant therapy should also be tested in the high-risk subgroup of haemodynamically unstable patients who need transfusions. This has been suggested by case reports of severe GI bleeding in Jehovah’s Witnesses who refused blood transfusions[20,21,25].
| 1. | Bieber E. Erythropoietin, the biology of erythropoiesis and epoetin alfa. An overview. J Reprod Med. 2001;46:521-530. [PubMed] |
| 2. | Larson B, Bremme K, Clyne N, Nordström L. Preoperative treatment of anemic women with epoetin beta. Acta Obstet Gynecol Scand. 2001;80:559-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Silverberg DS, Blum M, Agbaria Z, Deutsch V, Irony M, Schwartz D, Baruch R, Yachnin T, Steinbruch S, Iaina A. The effect of i.v. iron alone or in combination with low-dose erythropoietin in the rapid correction of anemia of chronic renal failure in the predialysis period. Clin Nephrol. 2001;55:212-219. [PubMed] |
| 4. | Gasche C, Waldhoer T, Feichtenschlager T, Male C, Mayer A, Mittermaier C, Petritsch W. Prediction of response to iron sucrose in inflammatory bowel disease-associated anemia. Am J Gastroenterol. 2001;96:2382-2387. [PubMed] |
| 5. | Allen UD, Kirby MA, Goeree R. Cost-effectiveness of recombinant human erythropoietin versus transfusions in the treatment of zidovudine-related anemia in HIV-infected children. Pediatr AIDS HIV Infect. 1997;8:4-11. [PubMed] |
| 6. | Atabek U, Alvarez R, Pello MJ, Alexander JB, Camishion RC, Curry C, Spence RK. Erythropoetin accelerates hematocrit recovery in post-surgical anemia. Am Surg. 1995;61:74-77. [PubMed] |
| 7. | Laine L, Peterson WL. Bleeding peptic ulcer. N Engl J Med. 1994;331:717-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 442] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 8. | Longstreth GF. Epidemiology of hospitalization for acute upper gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol. 1995;90:206-210. [PubMed] |
| 9. | Rockall TA, Logan RF, Devlin HB, Northfield TC. Incidence of and mortality from acute upper gastrointestinal haemorrhage in the United Kingdom. Steering Committee and members of the National Audit of Acute Upper Gastrointestinal Haemorrhage. BMJ. 1995;311:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 592] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 10. | Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38:316-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 920] [Article Influence: 30.7] [Reference Citation Analysis (1)] |
| 11. | Goodnough LT, Bach RG. Anemia, transfusion, and mortality. N Engl J Med. 2001;345:1272-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Lai KC, Hui WM, Wong BC, Ching CK, Lam SK. A retrospective and prospective study on the safety of discharging selected patients with duodenal ulcer bleeding on the same day as endoscopy. Gastrointest Endosc. 1997;45:26-30. [PubMed] |
| 13. | AuBuchon JP, Birkmeyer JD, Busch MP. Safety of the blood supply in the United States: opportunities and controversies. Ann Intern Med. 1997;127:904-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 112] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Goodnough LT, Brecher ME, Kanter MH, AuBuchon JP. Transfusion medicine. First of two parts--blood transfusion. N Engl J Med. 1999;340:438-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 600] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 15. | Kleinman S, Busch MP, Korelitz JJ, Schreiber GB. The incidence/window period model and its use to assess the risk of transfusion-transmitted human immunodeficiency virus and hepatitis C virus infection. Transfus Med Rev. 1997;11:155-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 115] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Wilhelm JA, Matter L, Schopfer K. The risk of transmitting cytomegalovirus to patients receiving blood transfusions. J Infect Dis. 1986;154:169-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Red blood cell transfusions contaminated with Yersinia enterocolitica--United States, 1991-1996, and initiation of a national study to detect bacteria-associated transfusion reactions. MMWR Morb Mortal Wkly Rep. 1997;46:553-555. [PubMed] |
| 18. | Welch HG, Meehan KR, Goodnough LT. Prudent strategies for elective red blood cell transfusion. Ann Intern Med. 1992;116:393-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 209] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Seidenfeld J, Piper M, Flamm C, Hasselblad V, Armitage JO, Bennett CL, Gordon MS, Lichtin AE, Wade JL, Woolf S. Epoetin treatment of anemia associated with cancer therapy: a systematic review and meta-analysis of controlled clinical trials. J Natl Cancer Inst. 2001;93:1204-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 144] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Pousada L, Fiorito J, Smyth C. Erythropoietin and anemia of gastrointestinal bleeding in a Jehovah's Witness. Ann Intern Med. 1990;112:552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Smith SN, Milov DE. Use of erythropoietin in Jehovah's Witness children following acute gastrointestinal blood loss. J Fla Med Assoc. 1993;80:103-105. [PubMed] |
| 22. | Koenig HM, Levine EA, Resnick DJ, Meyer WJ. Use of recombinant human erythropoietin in a Jehovah's Witness. J Clin Anesth. 1993;5:244-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Yazicioğlu L, Eryilmaz S, Sirlak M, Inan MB, Aral A, Taşöz R, Eren NT, Kaya B, Akalin H. Recombinant human erythropoietin administration in cardiac surgery. J Thorac Cardiovasc Surg. 2001;122:741-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Rutherford CJ, Schneider TJ, Dempsey H, Kirn DH, Brugnara C, Goldberg MA. Efficacy of different dosing regimens for recombinant human erythropoietin in a simulated perisurgical setting: the importance of iron availability in optimizing response. Am J Med. 1994;96:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Gannon CJ, Napolitano LM. Severe anemia after gastrointestinal hemorrhage in a Jehovah's Witness: new treatment strategies. Crit Care Med. 2002;30:1893-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
Edited by Wang XL Proofread by Zhu LH