INTRODUCTION
Complete and prompt liver regeneration after living liver donation and transplantation could occur in both donor and recipient in most circumstances[1-5]. Particularly in the transplant recipients, rejection[6,7] and ischemic injury[8] could result in inadequate regeneration, leading to hepatic insufficiency or overt liver failure. Early recognition and correction of these complications maximize the chance of liver graft salvage and good outcome for the patient.
Liver regeneration may be regulated cooperatively not only by humoral factors such as hormones, growth factors and growth inhibitory factors, but also by the immune system. It has been reported that regenerating hepatocytes became sensitive to the cytotoxic activity of normal liver-resident NK cells from partially hepatectomized mice[9-15]. It has also been reported that during the acute phase of regeneration after partial hepatectomy in rats, NK cell functions were temporary suppressed, followed by their recovery at the termination of regeneration[14], suggesting that such a selective suppression of NK cell functions during the acute phase represents an important regulatory mechanism for liver regeneration in the presence of hepatic NK cells. These observations indicate that liver-resident NK cells may be involved in regulating the extent of hepatocyte regeneration.
A recent study revealed that NKT cells proliferated in the liver after administration of IL-12[16], were cytotoxic effector cells and the main antimetastatic lymphocyte population in the liver. NKT cells are thought to be responsible for the recognition and regulation of not only malignant cell proliferation but also benign cell proliferation, suggesting that NKT cells may be involved in the regulation of hepatocyte regeneration[17].
It is accepted that both donor and recipient DCs mediate the rejection of graft in organ transplantation. Several studies have shown that immature donor DCs, deficient in surface costimulatory molecules, could induce T-cell hyporesponsiveness[18-20] and prolong graft survival in unmodified hosts[21-24]. Other studies showed that DCs treated with NF-κB decoy oligodeoxynuleoides (ODNs) containing specific NF-κB binding sites, which are maintained in an immature state, could induce tolerance of cardiac allograft[25,26]. Our recent study showed that NF-κB decoy ODNs-modified DCs could induce tolerance of rat liver allograft by promoting apoptosis of liver graft-infiltrating cells (GIC) in the portal areas and suppressing IFN-γ mRNA expression in the liver graft[27].
We hypothesize that the enhanced apoptosis of liver graft-infiltrating cells by NF-κB decoy ODNs-modified DCs can reduce the total number of liver-resident NK cells and suppress the cytotoxicity of NK cells to the regenerating hepatocytes, and consequently promote liver graft regeneration. In the present study we reported for the first time that NF-κB decoy ODNs-modified DCs could promote regeneration of partial liver allograft by suppressing hepatocyte apoptosis, liver-resident NK cell activity and IFN-γ production.
MATERIALS AND METHODS
NF-κB decoy ODNs
Double-stranded NF-κB decoy ODNs or scrambled ODNs (as a control for NF-κB decoy ODNs) were generated using equimolar amounts of single-stranded sense and antisense phosphorothioate-modified oligonucleotide containing two NF-κB binding sites (sense sequence 5’-AGGGACTTTCCGCTG-GGGACTTTCC-3’, NF-κB binding sites bold lines and underlined)[25] and scrambled oligonucleotide (sense sequence 5’-TTGCCGTACCTGACTTAGCC-3’)[28]. Sense and antisense strands of each oligonucleotide were mixed in the presence of 150 mM PBS, heated to 100 °C, and allowed to cool to room temperature to obtain double-stranded DNA.
Propagation of bone marrow-derived DC populations
Bone marrow cells harvested from femurs of normal SD rats were cultured in 24-well plates (2 × 106 per well) in 2 ml of RPMI 1640 complete medium supplemented with antibiotics, 10% fetal calf serum (FCS) and 4.0 ng/ml recombinant rat GM-CSF to obtain immature DCs. In addition to GM-CSF, 10 ng/ml recombinant rat IL-4 was added to cultures to obtain mature DCs. To select plates, 10 μM NF-κB decoy or scrambled ODNs was added at the initiation of culture of DCs[25]. Cytokine-enriched medium was refreshed every 2 days, after gentle swirling of the plates, half of the old medium was aspirated and an equivalent volume of fresh, cytokine-supplemented medium was added. Thus, nonadherent granulocytes were depleted without dislodging clusters of developing DCs attached loosely to a monolayer of plastic-adherent macrophages. Nonadherent cells released spontaneously from the clusters were harvested after 7 days.
Liver transplantation
Eighty male LEW rats and eighty male SD rats weighing 250-300 g were used in all the experiments. Allogeneic liver transplantations were performed using a combination of SD rats with LEW rats. All operations were performed under ether anesthesia in sterile conditions. Orthotopic 50% partial liver transplantations were performed according to the method described in our previous study[29]. Normal saline (group A), 1 × 107 GM-CSF-propagated DCs (group B), 1 × 107 GM-CSF + IL-4-propagated DCs (group C), and 1 × 107 GM-CSF + NF-κB decoy ODNs or scrambled ODNs-propagated DCs (group D or group E) were injected intravenously through the penile vein into recipient LEW rats 7 days prior to liver transplantation and immediately after liver transplantation, respectively. Liver graft samples and blood samples (n = 8) were harvested at 24 h, 48 h, 72 h and 84 h postoperatively. Part of the liver grafts was immediately used for isolation of NK cells.
Part of the liver graft tissues was immediately frozen in liquid nitrogen and kept at -80 °C for mRNA extraction and part of the liver graft tissues was preserved in 10% formalin for liver regeneration and apoptosis detection.
Liver allograft regeneration detection (BrdU labeling index)
Regeneration extent of liver allograft was indicated by BrdU labeling index of hepatocytes. The BrdU labeling index was determined as described previously. Briefly, BrdU administered intravenously at a dose of 50 mg/kg 30 min before death to measure DNA synthesis in the regenerative liver. Deparaffinized liver sections were incubated with anti-BrdU antibody for 60 min. Immunostaining for BrdU was performed by the avidin-biotin-immunoperoxidase method using ABC kit. A total of 1000 hepatocytes were counted and the labeling index was calculated.
Apoptosis of hepatocytes
Apoptotic cells in tissue sections were detected with the in situ cell death detection kit. The liver graft tissue sections were dewaxed and rehydrated according to standard protocols. Tissue sections were incubated with proteinase K(20 μg/ml in 10 mM Tris/HCl, pH 7.4-8.0) for 15 to 30 min at 21-37 °C. Endogenous peroxidase activity was quenched with blocking solution (0.3% H2O2 in methanol) for 30 min at room temperature before exposure to TUNEL reaction mixture at 37 °C for 60 min. After washed in stop wash buffer, POD (peroxidase) was added to react for 30 min at 37 °C. DAB-substrate was used for color development, and the sections were counterstained with Harrs’ hematoxylin. TUNEL staining was mounted under glass coverslip and analysed under light microscope.
Activity of liver graft-resident NK cells
Hepatic mononuclear cells (MNCs) were isolated by the sinusoidal lavage method of Bouwens and Wisse[30]. The cells were obtained by portal vein perfusion with 1% EDTA in PBS at 37 °C and a pressure of 50 H2O, and washed. Then, 30 mL of perfused fluid was collected from the inferior vena cava, and the erythrocytes and granulocytes were separated by the Ficoll (density 1.077) at 400 g for 25 min at 25 °C. NK activity was measured by the 51Cr release method. After washed with PBS, RPMI 1640 medium containing 10% fetal bovine serum was added and the cell count was adjusted to 1 × 106/ml. Next, the cultured K562 cells were harvested by centrifugation, 50 to 100 μl of 51Cr was added, and the mixture was incubated at 37 °C for 1 h. Also after the cells were washed with PBS, RPMI 1640 medium containing 10% fetal bovine serum was added and the cell count was adjusted to 1 × 106/ml. Then the target cells were placed in each well of a microplate. 1 N HCl was added to obtain maximum release and RPMI 1640 medium containing 10% fetal bovine serum was added to obtain spontaneous release (controls). Liver MNCs were added to the other wells at an effector /target ratio of 20. Centrifugation was performed for 5 min at 800 rpm, using a plate centrifuge, followed by incubation for 3.5 h in 5% CO2. The culture supernatant was collected from each well and radioactivity was measured with a g-scintillation counter. NK cells activity was calculated as follows:
Math 1
Semiquantitative RT-PCR assay for IFN-γ mRNA expression in liver graft
IFN-γ mRNA expression was determined by semiquantitative reverse transcription-PCR amplification in contrast with house-keeping gene β-actin. Total RNA from 10 mg liver allograft tissue was extracted using TripureTM reagent. First-strand cDNA was transcribed from 1 μg RNA using AMV and an Oligo (dT15) primer. PCR was performed in a 25 μl reaction system containing 10 μl cDNA, 2 μl 10 mM dNTP, 2.5 μl 10 × buffer, 2.5 μl 25 mmol.L-1 MgCl2, 2 μl specific primer, 5 μl water and 1 μl Taq (35 cycles: at 95 °C for 60 seconds, at 59 °C for 90 seconds, and at 72 °C for 10 seconds). The primers[31,32] used in PCR reactions were as follows: IFN-γ, 5’ primer 5’-AAGACAACCAGGCCATCAGCA-3’, 3’ primer 5’-AGCCACAGTGTGAGTTCAGTC-3’, to give a 547 bp product. β-actin, 5’ primer 5’-ATGCCATCCTGCGTCTGG ACCTGGC-3’, 3’ primer 5’-AGCATTTGCGGTGCACGAT GGAGGG-3’, to give a 607 bp product. PCR products of each sample were subjected to electrophoresis in a 15 g.L-1 agarose gel containing 0.5 mg.L-1 ethidium bromide. Densitometrical analysis using NIH image software was performed for semiquantification of PCR products, and mRNA expression was evaluated by the band-intensity ratio of IFN-γ to β-actin, and presented as percent of β-actin (%).
Statistical analysis
Statistic analysis of data was performed using t-test, P < 0.05 was considered statistically significant.
RESULTS
Augmented regeneration of partial liver allograft by NF-κB decoy ODNs-modified DCs
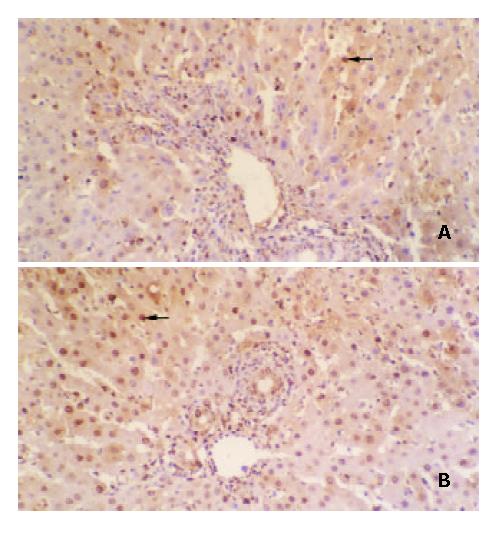
DNA synthesis by regenerating hepatocytes in the liver graft is shown in Figure 1. The BrdU labeling index of hepatocytes in liver graft peaked at postoperative 72 h in group A, group B and group E rats, whereas the BrdU labeling index of hepatocytes in liver graft peaked at postoperative 96 h in group C rats and 48 h in group D rats, respectively. The maximum BrdU labeling index of hepatocytes was 28.07 ± 4.53% for group A rats, 34.46 ± 6.72% for group B rats, 19.81 ± 3.65% for group C rats, 48.62 ± 9.07% for group D rats and 35.12 ± 6.37% for group E rats, respectively. The maximum BrdU labeling index of hepatocytes was significantly higher in group D rats but significantly lower in group C rats than that in the other groups rats (P < 0.01 or P < 0.001). The data suggested that regeneration of partial liver allograft was promoted by NF-κB decoy ODNs-modified DCs.
Figure 1 Augmented regeneration of partial liver allograft by NF-κB decoy ODNs-modified DCs (× 200).
BrdU-positive hepa-tocytes in the liver allografts from group C rats (A) and group D rats (B).
Suppression of apoptosis of hepatocytes by NF-κB decoy ODNs-modified DCs
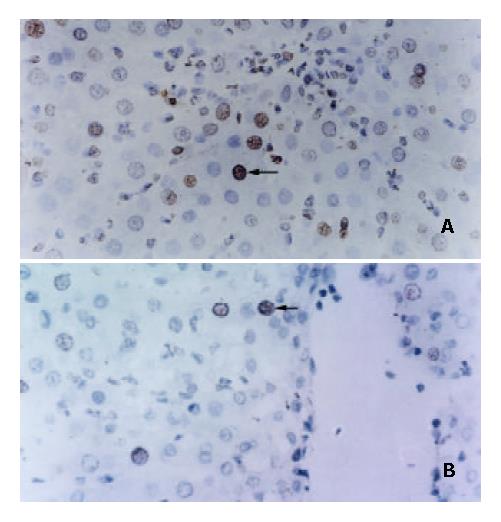
Some studies showed that liver regeneration was a process involving cell proliferation and inhibition of apoptosis[33-35]. To determine the augmented regeneration of liver allografts induced by NF-κB decoy ODNs-treated DCs was associated with the inhibited apoptotic death of hepatocytes, apoptotic activity in the liver graft at the time of maximal liver allograft regeneration was examined by TUNEL staining analysis. TUNEL staining of the liver graft sections revealed that the apoptosis index of hepatocytes was 13.07 ± 3.27% for group A rats, 9.32 ± 2.15% for group B rats, 17.76 ± 3.89% for group C rats, 5.42 ± 1.76% for group D rats and 9.75 ± 2.53% for group E rats, respectively. The apoptosis index of hepatocytes was significantly lower in group D rats but significantly higher in group C rats than that in the other groups rats (P < 0.05 or P < 0.01 or P < 0.001). These data strongly suggested that the augmented regeneration of the partial liver allograft induced by NF-κB decoy ODNs-modified DCs might be associated with the significantly suppressed apoptotic death of hepatocytes (Figure 2).
Figure 2 Suppression of apoptosis of hepatocytes in partial liver allografts by NF-κB decoy ODNs-modified DCs (× 400).
Apoptosis of hepatocytes in the liver allografts from group C rats (A) and group D rats (B).
Suppression of activity of liver allograft-resident NK cells by NF-κB decoy ODNs-modified DCs
Activity of the liver graft-resident NK cells at the time of the maximal liver allograft regeneration was measured with 51Cr release assay. A certain degree of activity of liver graft-resident NK cells was measured in group A rats. Activity of the liver graft-resident NK cells was partially suppressed by administration of immature DCs (group B and group E) but was significantly elevated by administration of IL-4 stimulated mature DCs (Group C), whereas the activity of the liver graft-resident NK cells was significantly suppressed by administration of NF-κB decoy ODNs-modified DCs (group D). The activity value of the liver graft-resident NK cells was 36.47 ± 6.32% for group A rats, 21.58 ± 4.61% for group B rats, 52.93 ± 7.73% for group C rats, 8.43 ± 2.18% for group D rats, and 23.06 ± 5.27% for group E rats, respectively. The activity of the liver graft-resident NK cells was significantly lower in group D rats but markedly higher in group C rats than that in the other groups rats (P < 0.001). The results suggested that the augmented regeneration of the partial liver allograft induced by NF-κB decoy ODNs-modified DCs might be associated with the markedly suppressed activity of the liver allograft-resident NK cells.
Suppression of IFN-γ production by NF-κB decoy ODNs-modified DCs
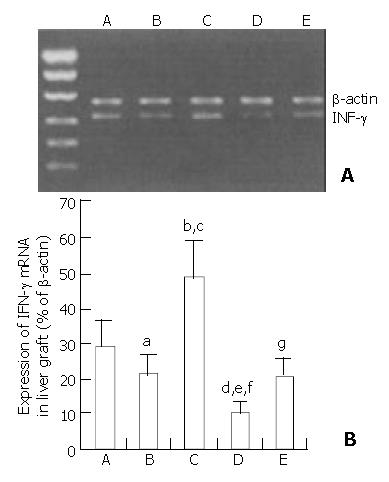
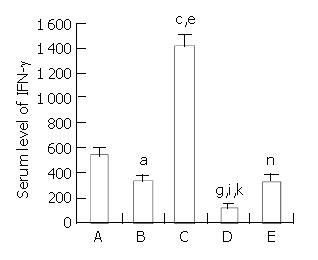
To determine the relationship of specific immunoregulator cytokine production to the regeneration of partial liver allograft, IFN-γ mRNA expression in the liver graft and serum level of IFN-γ at the time of the maximal liver allograft regeneration were examined by RT-PCR assay and ELISA(as shown in Figure 3 and Figure 4), respectively. A certain level of hepatic IFN-γ mRNA expression and a certain serum level of IFN-γ were detected in group A rats. Hepatic INF-γ mRNA expression and serum INF-γ production were partially down-regulated by administration of immature DCs (group B and group E) but were significantly up-regulated by administration of IL-4 stimulated mature DCs (Group C), whereas the hepatic INF-γ mRNA expression and serum INF-γ production were significantly suppressed by administration of NF-κB decoy ODNs-modified DCs (group D). The hepatic INF-γ mRNA expression level and the serum INF-γ level were markedly lower in group D rats but markedly higher in group C rats than that in the other groups rats. The results suggested that the augmented regeneration of the partial liver allograft induced by NF-κB decoy ODNs-modified DCs might be associated with the markedly suppressed hepatic IFN-γ mRNA expression and serum INF-γ production.
Figure 3 Suppression of IFN-γ mRNA expression in the liver allografts by NF-κB decoy ODNs-modified DCs.
aP < 0.05 vs group A, bP < 0.001 vs group A, cP < 0.001 vs group B, dP < 0.001 vs group A, eP < 0.001 vs group B, fP < 0.001 vs group C, gP > 0.05 vs group B.
Figure 4 Down-regulation of serum IFN-γ level in recipient rats by NF-κB decoy ODNs-modified DCs.
aP < 0.001 vs group A, cP < 0.001 vs group A , eP < 0.001 vs group B, gP < 0.001 vs group A, iP < 0.001 vs group B, kP < 0.001 vs group C, nP > 0.05 vs group B.
DISCUSSION
Our and other studies have shown that NF-κB decoy ODNs-modified immature DCs, which are stably deficient in surface costimulatory molecules and allosimulatory capacity, could inhibit alloantigen-specific T cell response and prolong cardiac and liver allograft survival in unmodified recipients[25-27]. NF-κB also has been found to be an important transcriptional regulator of liver regeneration[36-39]. NF-κB could prevent hepatocytes from undergoing apoptosis during development and liver regeneration, and inhibition of NF-κB activation could induce apoptosis but not proliferation of hepatocytes. Whether the immature DCs modified with NF-κB decoy ODNs, which inhibit NF-κB activation, can interfere with the liver allograft regeneration is unknown. To study the effect of NF-κB decoy ODNs-modified DCs on hepatic regeneration after liver transplantation, we measured the BrdU labeling index of hepatocytes in the partial liver allograft. Uchiyama et al[6] demonstrated that DNA synthesis in liver was slower after an orthotopic transplantation than after a partial hepatectomy. In our partial liver transplantation model, the BrdU labeling index of hepatocytes peaked at 72 h after a allogeneic transplantation, in contrast to the maximum DNA synthesis at 24 h that has been documented after a partial hepatectomy[40]. In the present study immature DCs or mature DCs were administered to recipient rats 7 days prior to liver transplantation and immediately after liver transplantation to detect whether NF-κB decoy ODNs-modified DCs could improve the delayed liver graft regeneration. Our results showed that partial liver graft DNA synthesis peaked earlier in group D rats (pretreatment with NF-κB decoy ODNs-modified immature DCs) but slower in group C rats (pretreatment with mature DCs) than that in the other groups rats. The maximum BrdU labeling index of hepatocytes in group D rats was significantly higher than that in the other groups rats, whereas the maximum BrdU labeling index of hepatocytes in group C rats was significantly lower than that in the other groups rats. In the present study, we also found that pretreatment with NF-κB decoy ODNs-modified DCs could reduce apoptosis of hepatocytes in the liver graft, however, pretreatment with mature DCs could promote apoptosis of hepatocytes in the liver graft. In situ TUNEL staining of the liver graft sections revealed that the maximum apoptosis of hepatocytes was detected in group C rats and the minimum apoptosis of hepatocytes was detected in group D rats. Our results suggested that NF-κB decoy ODNs-modified DCs could suppress apoptosis of hepatocytes and promote partial liver allograft regeneration.
The mechanisms by which NF-κB decoy ODNs-modified DCs promote regeneration of partial liver allograft remain to be clarified. In the present study, we found that the augmented regeneration of liver graft induced by NF-κB decoy ODNs-modified DCs might be associated with the reduced activity of liver-resident NK cells and the decreased IFN-γ production. A negative correlation was observed between liver regeneration and immune reaction (NK cell activity) in other studies[11,12], for the liver-resident and spleen-resident NK cells showed specific cytotoxicity against regenerating hepatocytes. Previous studies have shown that the liver grafts contained large numbers of NK cells and NK like cells with early lymphocyte activation before transplantation, and there was an influx of recipient NK and NK-like cells into the liver graft immediately after revascularization[41] and their cytotoxic activity was significantly augmented in the rejected liver allograft[42,43]. Other experimental studies have demonstrated that FasL expression on activated NK cells was augmented[44,45], and FasL ligation to Fas expressed on hepatocytes could mediate hepatocytes apoptosis[46,47]. Moreover, NK cells produce IFN-γ, which is involved in the aggravation of fulminant hepatitis. Thus, a decrease in NK cell activity may lead to reduction of IFN-γ. Although the definite role of NK cell activity and IFN-γ in liver regeneration is to be clarified, a rapid and profound suppression of a variety of spontaneous functions of NK cells in the liver or blood at the initiation phase of liver regeneration has been shown, including the rapidly decreased NK cell numbers and NK cell activity[11,12]. Vujanovic et al[14] found that depletion of NK cells by specific antibody accelerated rat liver regeneration after 70% partial hepatectomy. Tanigawa and Tamura[11,12] also found that FK506 and augmenter of liver regeneration promoted liver regeneration by reducing NK cell activity. Liu et al[9] recently suggested that the enhanced liver regeneration by oral ursodesoxycholic acid was mediated by suppression of NK cell activity in partially hepatectomized rats, and interleukin-2, a potent inducer of allograft rejection, could completely or partially block the decrease in NK activity and the increase in hepatocyte mitotic index. IFN-γ is secreted predominantly by activated T lymphocytes and activated NK cells interact in vitro in a complex network of mediators with other immune cytokines. IFN-γ is a potent stimulus for monocytes and macrophages that increases their expression of class II MHC antigens and Fc receptors. It also enhances TNF-α secretion. IFN-γ is also known to control the expression of mitochondrial transcription factor A, a nuclear gene responsible for mitochondrial metabolism. Moreover, IFN-γ could inhibit liver regeneration by stimulating MHC class II antigens expression by Kupffer cells in the regenerating liver[48]. These MHC class II antigen-positive Kupffer cells act as antigen-presenting cells and present hepatocyte as antigen, the so-called abnormal “self ”, to helper and cytotoxic T cells. Both types of T cells, in turn, may suppress hepatocyte proliferation. In this respect, a synergistic effect was also observed with the combination of IL-2 and IFN-γ. A recent study demonstrated that administration of IFN-γ inhibited liver regeneration by decreasing the mitochondrial transcription factor A expression[10]. These data suggested that augmentation of NK cell activity and IFN-γ production could inhibit liver regeneration. In the present study, we found that liver allograft-resident NK cell activity and hepatic IFN-γ mRNA expression and serum INF-γ production at the time of the maximal liver allograft regeneration were significantly suppressed by pretreatment with NF-κB decoy ODNs-modified immature DCs, whereas liver allograft-resident NK cell activity and hepatic IFN-γ mRNA expression and serum INF-γ production at the time of the maximal liver allograft regeneration were significantly elevated by pretreatment with mature DCs. The results suggested that there was a negative relationship between liver allograft regeneration and NK cell activity and IFN-γ production in the present study.
The mechanisms by which NF-κB decoy ODNs-modified immature DCs suppress the activity of liver allograft-resident NK cells and IFN-γ production after liver transplantation are still to be clarified. In our previous study we found in vitro and in vivo evidences that the stably immature NF-κB decoy ODNs-treated DCs could suppress T cell allostimulatory ability, promote apoptotic death of live graft-infiltrating lymphocytes, and consequently suppress Th1 immunostimulatory cytokines such as IL-2 and IFN-γ mRNA expression in the liver graft[27]. We hypothesized that the enhanced apoptosis of liver graft-infiltrating lymphocytes by NF-κB decoy ODNs-modified DCs could reduce the total number of the activated liver-resident NK cells and suppress the NK cell cytotoxicity to the regenerating hepatocytes. At the same time, the enhanced apoptosis of liver graft-infiltrating lymphocytes could decrease IFN-γ mRNA expression and IFN-γ production in the liver graft.
In summary, pretreatment with NF-κB decoy ODNs-modified immature DCs can suppress liver allograft-resident NK cell activity and IFN-γ production. The suppressed liver allograft-resident NK cell activity and IFN-γ production, in turn, may suppress hepatocyte apoptosis and promote regeneration of partial liver allograft.