INTRODUCTION
Colorectal cancer is one of the most common human malignancies. The genetic alterations in colorectal cancer may drive the transition of normal colorectal epithelium to adenomas and adenocarcinomas through increased proliferation and decreased cell death or apoptosis. Several peptide growth factors have been suggested as autocrine growth regulators in cancer cell lines[1]. Anomalous expression of growth factors and/or growth factor receptors, as well as abnormal response to growth factors and/or their receptors may be involved in cellular transformation. Among these growth factors, epidermal growth factor (EGF) is known to play a major role in regulation of cell proliferation. Epidermal growth factor has been shown to be a potent mitogen both in vitro and in vivo studies to stimulate DNA, RNA, and protein synthesis in the digestive tract[2,3].
Epidermal growth factor exerts its mitotic signal via a tyrosine kinase-type cell surface receptor, the EGF receptor (EGF-R). It has been reported that EGF-R is overexpressed in a number of human tumors[4-6]. The EGF-R level in patients with primary colorectal carcinoma ranged between 4 and 79 fmol/mg membrane protein (Kd = 0.1-0.4 × 10-9M)[7]. Messa et al[6] found that the expression of both EGF and EGF-R significantly increased in neoplastic tissues from patients with colorectal adenocarcinoma compared with that in their adjacent normal mucosa. The overexpression of EGF-R in human epidermoid carcinoma (A431) cells could allow selective growth advantage for tumor cells in the presence of normal or decreased ligand availability, and excessive ligand binding caused down-regulation of growth signaling and furthermore inhibition of growth and induction of apoptosis[4].
Epidermal growth factor affects cell proliferation by modulating the activity of cyclin-dependent kinase complex, a cell cycle regulator[8]. The cyclin-dependent kinase inhibitor p21 (also called WAF1, CAP20, Cip1, and Sdi1) may cause cell cycle arrest by both p53-dependent and -independent mechanisms[9]. EGF has shown to induce p21 expression in different human cancer cell lines[10-16].
Blockade of EGF signaling pathway by several methods affects the proliferation and/or apoptosis in a variety of tumor cell lines[4,17-19]. The EGF/EGF-R inhibitors may have potential for the therapy of tumors that are dependent on EGF-R signaling pathway for proliferation or survival. The purposes of this study were to investigate if EGF signaling inhibitors, EGF antibody and tyrphostin 51 (a tyrosine kinase inhibitor), mediated the action of EGF on apoptosis of human epithelial-type colorectal adenocarcinoma cells with EGF-R expression, and if these inhibitors affected the expression of EGF-R and p21, which are involved in EGF signaling pathway and cell cycle regulation, respectively.
MATERIALS AND METHODS
Cell line and treatments
Human colorectal adenocarcinoma cell line (SW480; BCRC No. 60249) was purchased from Bioresources Collection and Research Center (BCRC) at Food Industry Research and Development Institute (Hsinchu, Taiwan). Cells were grown in 900 mL/L Leibovitz’s L-15 medium with 100 mL/L fetal bovine serum at 37 °C without CO2. When cells reached 90% confluency, cells were switched to serum-free medium for 24 h to deplete growth factors in serum. Cells were then incubated with 0.6 mL/L dimethyl sulfoxide (DMSO, the control group), 225 ng/mL (37.5 nmol/L, a physiological concentration) EGF in 0.6 mL/L DMSO, 225 ng/mL EGF + 2.5 μg/mL (17 nmol/L) EGF antibody (Research and Diagnostics Systems, Inc., Minneapolis, MN) in 0.6 mL/L DMSO, or 225 ng/mL EGF + 215 ng/mL (0.8 μmol/L) tyrphostin 51 (a tyrosine kinase blocker to inhibit the EGF receptor, Sigma-Aldrich Co., St. Louis, MO) in 0.6 mL/L DMSO serum-free medium for 12 or 48 h. Cells and conditioned medium were collected. Protein contents in cells and conditioned medium were measured by the modified method of Lowry et al[20] using a Bio-Rad DC protein kit (Bio-Rad Laboratories, Hercules, CA).
Measurement of EGF in medium
The secretion of EGF into conditioned medium was measured by a commercial EGF immunoassay kit (QuantikineTM, Research and Diagnostics Systems, Inc.)[21]. Serum-free conditioned medium (200 μL) was incubated with murine monoclonal EGF antibody coated in a 96-well plate for 2 h at room temperature, washed 3 times with wash buffer, and then incubated with 200 µL polyclonal EGF antibody conjugated to horseradish peroxidase for 1 h. After several washes, samples were incubated with 200 µL substrate (tetramethylbenzidine:H2O2 = 1:1) for 20 min. The reaction was terminated by 50 μL of 1 mol/L sulfuric acid. The levels of EGF were determined at 450 nm and corrected at 540 nm using an ELISA reader (Multiskan RC, Thermo Labsystems, Helsinki, Finland).
Detection of apoptosis
Apoptosis was analyzed by fluorescence microscopy using an annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit (Oncogene Research Products, Boston, MA). The externalization of phosphatidylserine was as a marker of early-stage apoptosis using annexin V-FITC binding[22]. Cells (5 × 105) were suspended in 0.5 mL binding buffer (10 mmol/L Hepes, pH7.4/150 mmol/L NaCl/2.5 mmol/L CaCl2/1 mmol/L MgCl2/ 40 mg/L bovine serum albumin (BSA)), and incubated with 1.25 μL of 200 μg/mL annexin V-FITC in 50 mmol/L Tris (pH7.4), 0.1 mol/L NaCl, 10 mg/L BSA, and 0.2 mg/L NaN3 solution for 15 min in the dark. Cell suspension was centrifuged at 1000 g for 5 min, resuspended in 0.5 mL binding buffer, and mixed with 10 μL of 30 μg/mL propidium iodide in PBS. Samples were placed on ice in the dark and analyzed by fluorescence microscopy immediately.
Measurement of EGF receptor and p21 proteins
Cell suspension (30 μg total protein) pooled from 7 independent experiments (n = 7) was mixed with an equal volume of 2 × SDS-PAGE sample buffer (0.125 mol/L Tris-HCl, pH6.8/40 mg/L SDS/200 mL/L glycerol/100 mL/L 2-mercaptoethanol)[23], denatured at 100 °C for 3 min, and applied to SDS-PAGE (Bio-Rad Mini-PROTEAN 3 Cell, Bio-Rad Laboratories). Proteins were separated by 7.5% or 10% resolving gel for EGF receptor or p21, respectively, with 4% stacking gel in the running buffer (25 mmol/L Tris, pH8.3/192 mmol/L glycine/1 mg/L SDS) at 100 V for 1 h. After separation on the gel, proteins were then transferred onto the nitrocellulose membrane (0.45 μm) using a semi-dry transfer unit (Hoefer TE 70, Amersham Biosciences Ltd. Taiwan Branch, Taipei, Taiwan) in Towbin buffer (25 mmol/L Tris/192 mmol/L glycine/1.3 mmol/L SDS/100 mL/L methanol)[24] at 200 mA for 1 h. The membrane was washed briefly with PBS, and incubated with blocking buffer (50 mg/L skim milk/1 mL/L Tween-20 in PBS) for 1 h. After blocking, the membrane was incubated with 1 μg/mL mouse anti-human phosphorylated EGF receptor (eps15, BD Transduction Laboratories, San Diego, CA), p21 (p21Cip1/WAF1, BD Transduction Laboratories), or α-tubulin (TU-02, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) antibody at room temperature for 1 h. The membrane was washed 3 times with wash buffer (1 mL/L Tween-20 in PBS), and incubated with 10 μg/mL goat anti-mouse IgG-horseradish peroxide conjugate (Leinco Technologies, Inc., St. Louis, MO,) for 1 h. The blot was washed again 3 times with wash buffer, incubated with ECLTM Western blotting detection reagents (Amersham Biosciences Ltd. Taiwan Branch) for 1 min, and exposed to an X-ray film for 15 s. The bands were quantitated by an image analysis system (Gel analysis system, EverGene Biotechnology, Taipei, Taiwan) and Phoretix 1D Lite software (Phoretix International Ltd., Newcastle upon Tyne, UK).
Statistical analysis
Data are expressed as mean ± SD. Data were analyzed by one-way ANOVA to determine the treatment effect using SAS (version 6.12, SAS Institute Inc., Cary, NC). Fisher‘s least significant difference test was used to make post-hoc comparisons if the main effect was demonstrated. Differences were considered significant at P < 0.05.
RESULTS
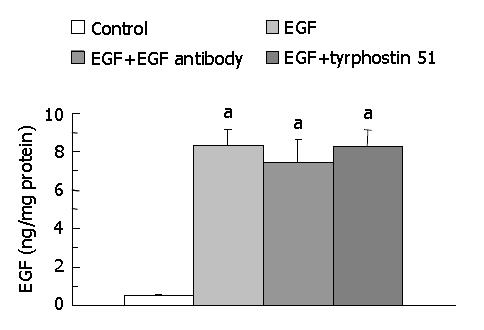
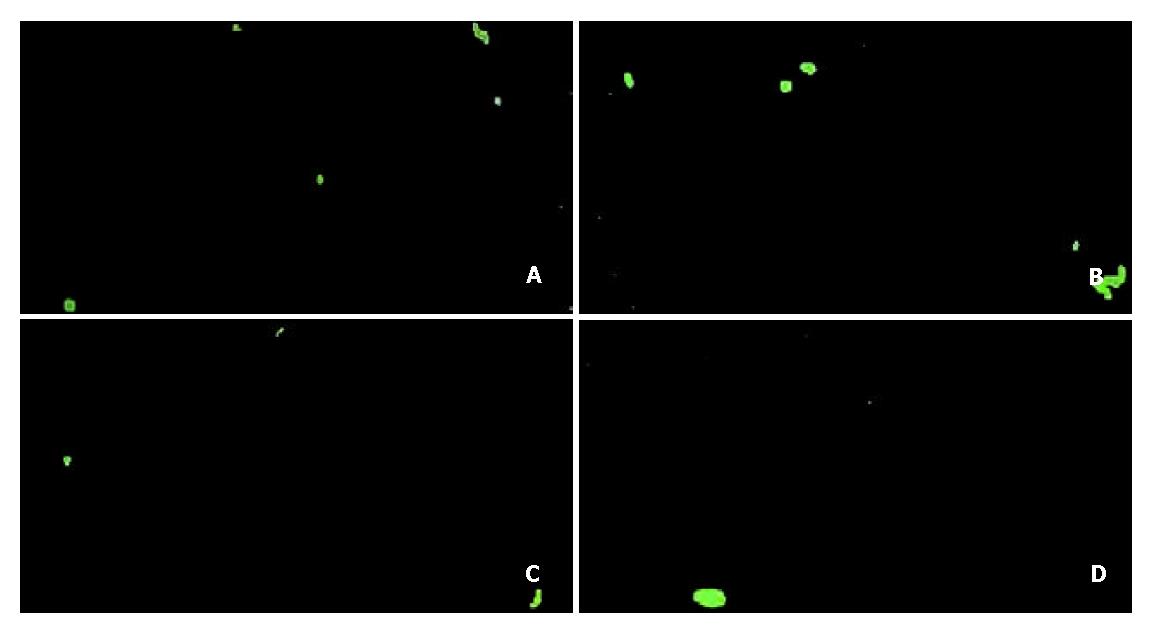
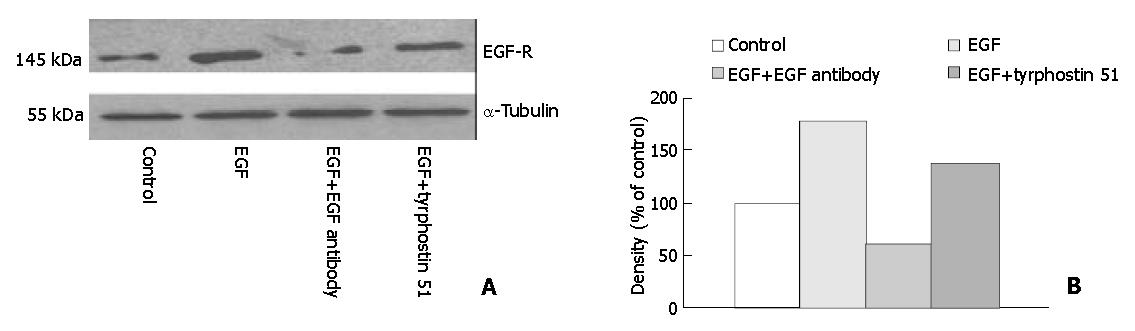
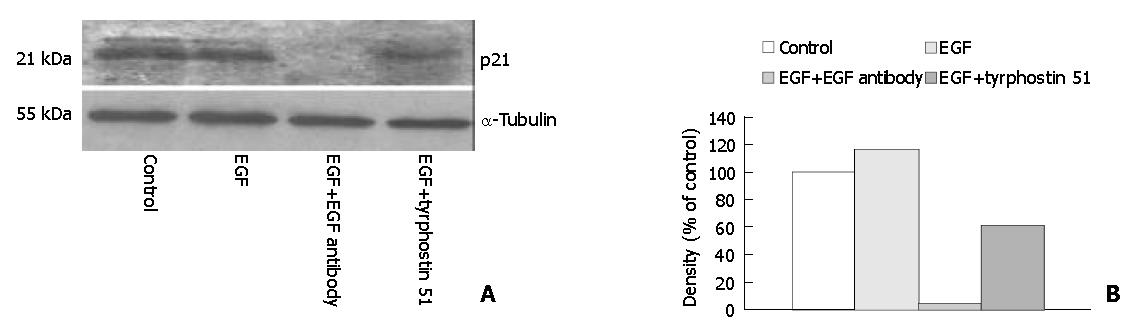
The secretion of EGF into medium was significantly higher (P < 0.05) in the EGF (8.35 ± 0.82 ng/mg protein, n = 7), EGF + EGF antibody (7.44 ± 1.17 ng/mg protein, n = 7), and EGF + tyrphostin 51 (8.28 ± 0.84 ng/mg protein, n = 7) groups than that in the control group (0.49 ± 0.05 ng/mg protein, n = 7) (Figure 1). However, the level of EGF did not significantly differ among the EGF-treated groups. Annexin V-FITC apoptosis detection assay showed that FITC-positive cells were significantly more often found (P < 0.05) in the control (5.0 ± 1.8/field) (Figure 2A) and EGF (6.0 ± 1.3/field) (Figure 2B) groups, with a range of 2 to 8 positive cells per field ( × 100), compared with those in the EGF + EGF antibody (2.1 ± 1.3/field) (Figure 2C) and EGF + tyrphostin 51 (1.9 ± 1.3/field) (Figure 2D) groups, with a range of 0 to 4 cells per field ( × 100) after 12 h treatments. However, the numbers of apoptotic cells did not significantly differ between the control and EGF groups and between the EGF + EGF antibody and EGF + tyrphostin 51 groups. The expression of phosphorylated EGF-R in the EGF, EGF + EGF antibody, and EGF + tyrphostin 51 groups was 176.8%, 62.4%, and 138.1% of the control group, respectively (Figure 3). The expression of p21 protein in the EGF, EGF + EGF antibody, and EGF + tyrphostin 51 groups was 115.7%, 4.8%, and 61.5% of the control group, respectively (Figure 4).
Figure 1 Levels of EGF in medium after incubation of SW480 cells with 0.
06% dimethyl sulfoxide (DMSO, the control group), 225 ng/mL (37.5 nmol/L) EGF in 0.6 mL/L DMSO, 225 ng/mL EGF + 2.5 μg/mL (17 nmol/L) EGF antibody in 0.6 mL/L DMSO, or 225 ng/mL EGF + 215 ng/mL (0.8 μmol/L) tyrphostin 51 in 0.6 mL/L DMSO serum-free medium for 48 h. Data are expressed as mean ± SD (n = 7). aP < 0.05 significantly different between the control and EGF-treated groups.
Figure 2 Apoptotic cells stained by annexin V-FITC binding (green) after incubation of SW480 cells with 0.
6 mL/L dimethyl sulfoxide (DMSO, the control group) (A), 225 ng/mL (37.5 nmol/L) EGF in 0.6 mL/L DMSO (B), 225 ng/mL EGF + 2.5 μg/mL (17 nmol/L) EGF antibody in 0.6 mL/L DMSO (C), or 225 ng/mL EGF + 215 ng/mL (0.8 μmol/L) tyrphostin 51 in 0.6 mL/L DMSO serum-free medium (D) for 12 h. MicrograpHmagnified by × 100 is the representative of seven independent experiments (n = 7).
Figure 3 Expression of phosphorylated EGF receptor (EGF-R) with the molecular weight of 145 kDa visualized by Western blotting (A) and quantitated by an image analysis system (B) after incubation of SW480 cells with 0.
6 mL/L dimethyl sulfoxide (DMSO, the control group), 225 ng/mL (37.5 nmol/L) EGF in 0.6 mL/L DMSO, 225 ng/mL EGF + 2.5 μg/mL (17 nmol/L) EGF antibody in 0.6 mL/L DMSO, or 225 ng/mL EGF + 215 ng/mL (0.8 μmol/L) tyrphostin 51 in 0.6 mL/L DMSO serum-free medium for 48 h. Samples were pooled from 7 independent experiments (n = 7). Density was calibrated by an internal control, α-tubulin (55 kDa).
Figure 4 Expression of p21 protein with the molecular weight of 21 kDa visualized by Western blotting (A) and quantitated by an image analysis system (B) after incubation of SW480 cells with 0.
6 mL/L dimethyl sulfoxide (DMSO, the control group), 225 ng/mL (37.5 nmol/L) EGF in 0.6 mL/L DMSO, 225 ng/mL EGF + 2.5 μg/mL (17 nmol/L) EGF antibody in 0.6 mL/L DMSO, or 225 ng/mL EGF + 215 ng/mL (0.8 μmol/L) tyrphostin 51 in 0.6 mL/L DMSO serum-free medium for 48 h. Samples were pooled from 7 independent experiments (n = 7). Density was calibrated by an internal control, α-tubulin (55 kDa).
DISCUSSION
Our data showed that EGF treatment at a dose of 225 ng/mL (37.5 nmol/L) did not further stimulate apoptosis compared with the control group, but significantly increased to nearly 2-fold the expression of phosphorylated EGF-R. Gulli et al[4] demonstrated that exogenous EGF (60 ng/mL; 10 nmol/L) inhibited cell proliferation and induced morphological features of apoptosis in A431 cells with overexpressed EGF-R by induction of a 15-fold increase in EGF-R autophosphorylation, which down-regulated EGF signal transduction. At a lower concentration of EGF (0.06 ng/mL, 10 pmol/L), EGF-R autophosphorylation and cell proliferation increased to 2-fold compared with those in untreated cells[4]. Previous studies showed the expression of EGF-R on the cell surface affected the action of EGF[15,25]. A431 cells grown as three-dimensional spheroids showed growth stimulation in response to nanomolar concentrations of EGF, while monolayer cultures showed growth inhibition[25]. The expression of EGF-R on monolayers of A431 cells was 20-fold greater than that on three-dimensional spheroids. Autophosphorylation of the EGF-R also increased in response to EGF in monolayer cultures of A431 cells. However, EGF prevented apoptosis of human bladder carcinoma 647V cells cultured as three-dimensional spheroids with lower expression of EGF-R[15]. The data indicated that EGF could play a dual role in modulation of apoptosis depending on the level of EGF-R. The overexpression of EGF-R down-regulated growth-stimulating action of EGF when cells were grown in monolayer cultures.
Apoptosis occurred prior to and in response to EGF treatment in SW480 cells. Previous studies found that cell lines, such as A431[4] and human mammary adenocarcinoma MDA-MB-468[26] cells, which overexpress the EGF-R underwent apoptosis in response to EGF treatment. Additionally, apoptosis was greatly enhanced when cells were growth-arrested prior to EGF treatment[26]. Our study showed that apoptosis was also induced in the control group, probably because cells were arrested after 24 h incubation in serum-free medium. However, apoptosis was induced to occur but not further enhanced by EGF compared with that without EGF (the control group) within 12 h. EGF-induced apoptosis may involve activation of activator protein-1[26], inhibition of nuclear transcriptional factor-kappaB (NF-κB)[27] and protein kinase B (PKB/Akt) activation[28], and cell detachment (a decline in cell adhesion)[29,30].
Compared with the EGF group, apoptosis was inhibited after EGF signal transduction was blocked by EGF antibody or tyrosine kinase inhibitor. The expression of phosphorylated EGF-R and p21 decreased in the groups treated with EGF signaling inhibitors, especially in the EGF + EGF antibody group. Our data indicated that the inhibition of EGF signal transduction by EGF antibody or by EGF-R inhibitor decreased apoptosis of human colorectal adenocarcinoma cells, at least in part, through regulation of EGF-R and p21. SW480 cells produced the most transforming growth factor (TGF)β-like rather than TGFα-like activity and had no measurable TGFβ membrane receptors, but EGF receptors were detectable[31]. Additionally, the concentration of endogenous EGF detectable in conditioned medium of the control group was much lower than that of exogenous EGF in the EGF-treated groups. Therefore, apoptosis of SW480 cells was primarily mediated by exogenous EGF and EGF signaling inhibitors through EGF-R signaling pathway in a paracrine rather than an autocrine manner. Previous studies showed that inhibition of EGF signal transduction could reverse the action of EGF on cell proliferation[4,17-19]. Anti-sense EGF-R RNA down-regulated the proliferation[17] and invasive properties[18] of human colon tumor cells. When A431 cells were simultaneously treated with EGF (60 ng/mL, 10 nmol/L) and EGF antibody (15 μg/mL), a significant reduction in EGF-R autophosphorylation reversed the action of EGF on cell proliferation[4]. Tyrphostin, the most potent EGF-R kinase inhibitor, inhibited EGF-dependent proliferation of A431/clone 15 cells, but with little or no effect on EGF-independent cell growth[32]. The phosphorylation of EGF-R was decreased by an inhibitor of EGF-R tyrosine kinase, RG-13022 (α-(3’-pyridyl)-3,4-dimethoxy)cinnamonitrile), in A431 cells[4]. Additionally, another tyrosine kinase inhibitor, CP-358,774 ([6,7-bis(2-methoxy-ethoxy)-quinazolin-4-yl]-(3-ethynylphenyl)amine), inhibited proliferation and triggered apoptosis through arrest of cell cycle progression in the G1 phase by accumulation of p27KIP1, a mitotic inhibitor, in human colorectal carcinoma (DiFi) cells[19].
Similar to our findings, exposure to EGF at a nanomolar concentration reduced DNA synthesis, arrested cells in the G0/G1 phase, and elevated p21 protein in human squamous carcinoma cells, indicating that p21 plays a role in mediating EGF-induced growth inhibition[10-12]. Additionally, EGF-mediated growth inhibition was associated with induction of p21 in human breast[13,14], bladder[15], and esophageal[16] cancer cells. EGF-increasing p21 expression was by stabilization of p21 at the post-transcriptional and post-translational levels[12], and activation of signal transducer and activator of transcription (STAT)1 and STAT3[13,16,33]. Consistent with our study, EGF-induced p21 expression was inhibited by the EGF-R tyrosine kinase inhibitor, tyrphostin AG1478 in A431 cells[10].
In conclusion, EGF antibody and tyrphostin 51 can inhibit the action of EGF on apoptosis in human colorectal cancer SW480 cells through down-regulation of EGF receptor and p21 expression. Furthermore, EGF at a higher concentration may have potential for the therapy of tumors with overexpressed EGF-R, which is dependent on EGF-R signaling pathway for proliferation.