Published online Feb 15, 2004. doi: 10.3748/wjg.v10.i4.481
Revised: January 2, 2004
Accepted: January 8, 2004
Published online: February 15, 2004
AIM: Pituitary tumor transforming gene (PTTG1) is overexpressed in a variety of tumors, including carcinomas of the lung, breast, colon, as well as in leukemia, lymphoma and pituitary adenomas. However, there is little information on its expression in gastric carcinoma. We sought to investigate the expression of PTTG1 in gastric carcinoma and to explore the relationship between its expression and clinicopathological factors.
METHODS: We studied 75 primary human gastric adenocarcinomas, including 17 mucosal carcinomas, 21 submucosal infiltrative carcinomas, 12 carcinomas invading proprial muscle layers, 6 carcinomas reaching the subserosa, and 19 carcinomas penetrating the serosal surface. Immunohistochemical analysis was performed using paraffin-embedded sections of gastric adenocarcinomas.
RESULTS: PTTG1 was expressed heterogeneously in carcinomas. Positive PTTG1 staining was observed in 65.3% of the carcinomas (49 of 75). Its expression did not correlate significantly with either the histological type or the depth of infiltration of the gastric carcinomas. However, a statistical analysis showed significant differences between the primary adenocarcinomas and the associated metastatic lymph nodes.
CONCLUSION: The results of this study demonstrate that PTTG1 expression is enhanced in metastatic lymph nodes in comparison to that in primary carcinomas. We suggest that PTTG1 may contribute to lymph node metastases in gastric carcinoma.
- Citation: Wen CY, Nakayama T, Wang AP, Nakashima M, Ding YT, Ito M, Ishibashi H, Matsuu M, Shichijo K, Sekine I. Expression of pituitary tumor transforming gene in human gastric carcinoma. World J Gastroenterol 2004; 10(4): 481-483
- URL: https://www.wjgnet.com/1007-9327/full/v10/i4/481.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i4.481
Gastric carcinoma is one of the most common causes of malignancy-related death worldwide. Recent molecular biological studies suggest that genetic instability may play an important role in the pathogenesis of gastric carcinogenesis[1]. There are at least two distinct genetic instabilities in gastric tumorigenesis. One is chromosomal instability and the other is instability of the microsatellites. In the former, diminished expression of tumor suppressor genes, such as p53, Rb, APC, MCC and DCC, plays an important role in carcinogenesis. Whereas in the latter, the defective repair of mismatched bases results in an increase in the rate of point mutations[2-4].
Aneuploidy is a numerical imbalance in chromosomes caused by missegregation during cell division. A critical event that promotes equal partitioning of chromosomes during mitosis is the proper and timely separation of sister chromatids attached to each other and to the mitotic spindle. Interestingly, pituitary tumor transforming gene (PTTG1) is a securin that acts as an inhibitor of chromatid separation[5].
PTTG1 is a novel oncogene that has been identified by Pei et al[6]. PTTG1 overexpression in mouse fibroblasts and NIH 3T3 cells induced cellular proliferation and transformation, both in vitro and in vivo[6,7]. PTTG1 is overexpressed in a variety of tumors, including carcinomas of the lung, breast, colon, as well as in leukemia, lymphoma and pituitary adenomas[8-11]. The expression of PTTG1 in normal tissuses is restricted with the highest expression occurring in the testis[6,8,9]. PTTG1 is expressed in a stage-specific manner in germ cells during the spermatogenic cycle, suggesting that it may play a role in male germ cell differentiation[12]. PTTG1 also regulates the secretion of basic fibroblast growth factor[8].
In the present study, we investigated PTTG1 expression and explored the relationship between its expression and clinicopathological factors in 75 gastric carcinoma specimens.
We studied 75 primary human gastric adenocarcinomas, including 17 mucosal carcinomas, 21 submucosal infiltrative carcinomas, 12 carcinomas invading proprial muscle layers, 6 carcinomas reaching the subserosa, and 19 carcinomas penetrating the serosal surface. All tumor specimens were obtained from patients at the Nagasaki University Hospital between 2001 and 2002. Each tumor was assigned a histological type and a depth grading of infiltration according to the Japanese Classification of Gastric Carcinoma by the Japanese Gastric Cancer Association[13]. The primary human gastric adenocarcinomas were classified histologically as follows: 4 papillary adenocarcinomas, 18 tubular adenocarcinomas of the well differentiated type, 22 tubular adenocarcinomas of the moderately differentiated type, 7 poorly differentiated adenocarcinomas of the solid type, 10 poorly differentiated adenocarcinomas of the nonsolid type, 12 signet-ring cell carcinomas, and 2 mucinous adenocarcinomas. Diagnosis was established by two independent pathologists (CY Wen and T Nakayama) and cases of questionable diagnosis were omitted from the study.
Formalin-fixed and paraffin-embedded tissues were cut into 4 μm sections, deparaffinized in xylene, and rehydrated in phosphate-buffered saline. Deparaffinized sections were subsequently preincubated in 3% H2O2 for 30 min, followed by incubation in normal bovine serum to prevent nonspecific binding and subsequently incubated overnight at 4 °C in a primary polyclonal antibody directed against human PTTG1 (2 μg/ml) (Zymed Laboratories, Inc. South San Francisco, CA, USA). Next, the slides were incubated in biotinylated anti-rabbit immunoglobulin G followed by avidin-horseradish peroxidase and the reaction product was resolved using diaminobenzidine (DAB) (Vectastain ABC kit; Vector Laboratories, Burlingame, CA, USA). For the PTTG1 expression, pituitary adenoma served as a positive control, and the slide with the primary antibody omitted was used as a negative control. Analysis of the immunohistochemical staining was performed by two investigators (CY Wen and T Nakayama). PTTG1 expression was classified into three categories depending on the percentage of cells stained and/or the intensity of staining: -, 0% to 10% positive tumor cells; +, 10% to 50% positive tumor cells; and ++, > 50% positive tumor cells.
Statistical analyses were performed using Spearman’s correlation coefficient by rank test and the Mann-Whitney U test. A P value < 0.05 was accepted as statistically significant.
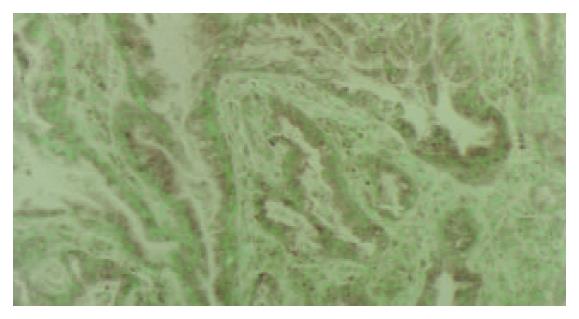
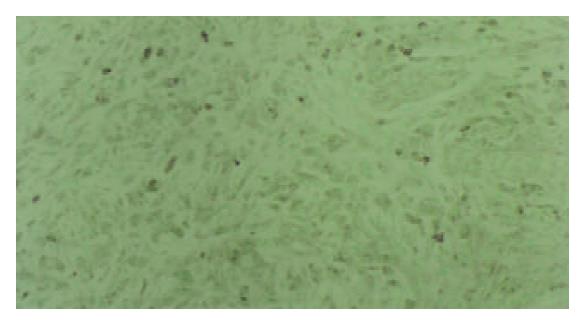
PTTG1 protein was detected in the cytoplasm. Benign gastric epithelia showed focal and patchy immunoreactivity of PTTG1 with faint to mild staining intensity. The results of immunohistochemical analysis are summarized in Table 1. PTTG1 was expressed heterogeneously in carcinomas. Positive PTTG1 staining was observed in 65.3% of carcinomas (in 49 out of 75). The correlation between PTTG1 immunoreactivity and tumor histological type is shown in Table 1. Tubular adenocarcinomas of the well and moderately differentiated types were stained strongly for PTTG1 (Figure 1). However, poorly differentiated adenocarcinomas of the solid and nonsolid types exhibited weak PTTG1 expression (Figure 2) and mucinous adenocarcinomas were negative for antibody reaction.
| n | - | + | ++ | P value | |
| Total carcinomas | 75 | 26(34.7) | 31(41.3) | 18(24.0) | |
| Papillary adenocarcinomas | 4 | 1(25.0) | 2(50.0) | 1(25.0) | |
| Tubular adenocarcinomas | |||||
| Well differentiated | 18 | 6(33.3) | 6(33.3) | 6(33.3) | |
| Moderately differentiated | 22 | 7(31.8) | 7(31.8) | 8(36.4) | |
| Poorly differentiated adenocarcinomas | |||||
| Solid types | 7 | 4(40.0) | 2(28.6) | 1(14.3) | |
| Nonsolid type | 10 | 4(40.0) | 6(60.0) | 0(0.0) | |
| Signet-ring cell carcinoma | 12 | 4(33.3) | 7(58.3) | 1(8.3) | |
| Mucinous adenocarcinoma | 2 | 2(100.0) | 0(0.0) | 0(0.0) | > 0.05 |
The relationship between immunoreactivity and the depth of tumor infiltration is shown in Table 2. PTTG1 staining was positive for tumors with mucosal infiltration (74.7%) or submucosal infiltration (76.2%), whereas expression was markedly weaker in tumors infiltrating proprial muscle layers, subserosa, and serosal surface. PTTG1 expression did not correlate with either histological type or depth of infiltration of gastric carcinomas (Table 1,Table 2).
| n | - | + | ++ | P value | |
| m | 17 | 6(35.3) | 5(29.4) | 6(35.3) | |
| sm | 21 | 5(23.8) | 11(52.4) | 5(23.8) | |
| mp | 12 | 5(41.7) | 4(33.3) | 3(25.0) | |
| ss | 6 | 2(33.3) | 4(66.7) | 0(0.0) | |
| se | 19 | 8(42.1) | 7(36.8) | 4(21.1) | >0.05 |
| Total | 75 | 26(34.7) | 31(41.3) | 18(24.0) |
PTTG1 expression in primary adenocarcinomas and metastatic lymph nodes are compared in Table 3. PTTG1 staining was mostly negative for the primary sites, i.e., 7 out of 19 tumors were positive (36.8%), whereas 79% of the metastatic sites were immunoreactive (15 out of 19). Statistical analysis showed a significant difference in staining intensity between primary adenocarcinomas and metastatic lymph nodes (P < 0.005, Table 3).
| n | - | + | ++ | P value | |
| Primary adenocarcinomas | 19 | 12(63.2) | 7(36.8) | 0(0.0) | |
| Metastatic lymph nodes | 19 | 4(21.0) | 9(47.2) | 6(31.6) | < 0.005 |
It is now widely accepted that cancer results from the accumulation of mutations in genes that regulate cell birth or death. An underlying genetic instability might be a prerequisite for the generation of multiple mutations that lead to cancer[14,15]. For example, losses or gains in the number of whole chromosomes (aneuploidy) have been found in nearly all major human tumor types, including gastric cancers[16,17].
A large number of potential targets made putative cancer cells susceptible to several mechanisms that may lead to chromosome instability[18]. The finding that PTTG1 codes for a securin involved in regulating chromatid separation during cell division suggested that securin overexpression might disrupt cell division, generate chromosomal instability and, thereby, increase cell susceptibility to mutations during subsequent divisions[5].
In the present study, we investigated the correlation between PTTG1 expression and histological classification, invasive depth and lymph node metastasis in gastric carcinomas. PTTG1 expression was enhanced in metastatic lymph nodes in comparison to that found in primary carcinomas. Shibata et al[19] recently showed a significant correlation between high PTTG1 expression, advanced pathological stage of cancer and lymph node metastasis in esophageal cancer. Heaney et al[10] reported that high PTTG1 expression correlated with extension to the bowel wall, metastasis, vascularity and Dukes’ staging. These findings suggest that enhanced PTTG1 expression may be a marker for invasive carcinoma.
Pei[20] recently reported that PTTG1 could bind to the c-myc promoter near the transcription initiation site and activate c-myc transcription in transfected cells. These findings suggest that PTTG1 may act as a transcription activator and may be involved in cellular transformation and tumorigenesis via activation of the c-myc oncogene. Furthermore, PTTG1 induces angiogenesis, a key determinant and rate-limiting step in tumor progression and metastatic spread, through basic fibroblast growth factor. Poor prognosis in patients exhibiting high PTTG mRNA expression might reflect an angiogenic phenotype in addition to the acquisition of chromosome instability[6,21].
In conclusion, the results of this study demonstrate that PTTG1 expression is enhanced in metastatic lymph nodes in comparison to that in primary carcinomas. We suggest that PTTG1 is a marker of lymph node metastasis in gastric carcinomas.
| 1. | Fang DC, Jass JR, Wang DX, Zhou XD, Luo YH, Young J. Infrequent loss of heterozygosity of APC/MCC and DCC genes in gastric cancer showing DNA microsatellite instability. J Clin Pathol. 1999;52:504-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Fang DC, Yang SM, Zhou XD, Wang DX, Luo YH. Telomere erosion is independent of microsatellite instability but related to loss of heterozygosity in gastric cancer. World J Gastroenterol. 2001;7:522-526. [PubMed] |
| 3. | Martins C, Kedda MA, Kew MC. Characterization of six tumor suppressor genes and microsatellite instability in hepatocellular carcinoma in southern African blacks. World J Gastroenterol. 1999;5:470-476. [PubMed] |
| 4. | Wu BP, Zhang YL, Zhou DY, Gao CF, Lai ZS. Microsatellite instability, MMR gene expression and proliferation kinetics in colorectal cancer with famillial predisposition. World J Gastroenterol. 2000;6:902-905. [PubMed] |
| 5. | Zou H, McGarry TJ, Bernal T, Kirschner MW. Identification of a vertebrate sister-chromatid separation inhibitor involved in transformation and tumorigenesis. Science. 1999;285:418-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 589] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 6. | Pei L, Melmed S. Isolation and characterization of a pituitary tumor-transforming gene (PTTG). Mol Endocrinol. 1997;11:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 347] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 7. | Kakar SS, Jennes L. Molecular cloning and characterization of the tumor transforming gene (TUTR1): a novel gene in human tumorigenesis. Cytogenet Cell Genet. 1999;84:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Zhang X, Horwitz GA, Prezant TR, Valentini A, Nakashima M, Bronstein MD, Melmed S. Structure, expression, and function of human pituitary tumor-transforming gene (PTTG). Mol Endocrinol. 1999;13:156-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 193] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Domínguez A, Ramos-Morales F, Romero F, Rios RM, Dreyfus F, Tortolero M, Pintor-Toro JA. hpttg, a human homologue of rat pttg, is overexpressed in hematopoietic neoplasms. Evidence for a transcriptional activation function of hPTTG. Oncogene. 1998;17:2187-2193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 136] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Heaney AP, Singson R, McCabe CJ, Nelson V, Nakashima M, Melmed S. Expression of pituitary-tumour transforming gene in colorectal tumours. Lancet. 2000;355:716-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 165] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Sáez C, Japón MA, Ramos-Morales F, Romero F, Segura DI, Tortolero M, Pintor-Toro JA. hpttg is over-expressed in pituitary adenomas and other primary epithelial neoplasias. Oncogene. 1999;18:5473-5476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 104] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Pei L. Genomic organization and identification of an enhancer element containing binding sites for multiple proteins in rat pituitary tumor-transforming gene. J Biol Chem. 1998;273:5219-5225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Japanese Gastric Cancer Association: Japanese Classification of Gastric Carcinoma (The 13th Edition). Edited by Japanese Gastric Cancer Association, Tokyo, Kenehara Press 1999. . |
| 14. | Loeb LA. Mutator phenotype may be required for multistage carcinogenesis. Cancer Res. 1991;51:3075-3079. [PubMed] |
| 15. | Hartwell L. Defects in a cell cycle checkpoint may be responsible for the genomic instability of cancer cells. Cell. 1992;71:543-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 555] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 16. | Furuya T, Uchiyama T, Murakami T, Adachi A, Kawauchi S, Oga A, Hirano T, Sasaki K. Relationship between chromosomal instability and intratumoral regional DNA ploidy heterogeneity in primary gastric cancers. Clin Cancer Res. 2000;6:2815-2820. [PubMed] |
| 17. | Choma D, Daurès JP, Quantin X, Pujol JL. Aneuploidy and prognosis of non-small-cell lung cancer: a meta-analysis of published data. Br J Cancer. 2001;85:14-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2881] [Cited by in RCA: 2833] [Article Influence: 101.2] [Reference Citation Analysis (0)] |
| 19. | Shibata Y, Haruki N, Kuwabara Y, Nishiwaki T, Kato J, Shinoda N, Sato A, Kimura M, Koyama H, Toyama T. Expression of PTTG (pituitary tumor transforming gene) in esophageal cancer. Jpn J Clin Oncol. 2002;32:233-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Pei L. Identification of c-myc as a down-stream target for pituitary tumor-transforming gene. J Biol Chem. 2001;276:8484-8491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 112] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Zetter BR. Angiogenesis and tumor metastasis. Annu Rev Med. 1998;49:407-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 675] [Cited by in RCA: 703] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
Edited by Wang XL Proofread by Zhu LH