Published online Feb 1, 2004. doi: 10.3748/wjg.v10.i3.449
Revised: September 14, 2003
Accepted: September 20, 2003
Published online: February 1, 2004
AIM: The effects of cobalt, copper, cadmium and barium ions on the cysts of Entamoeba histolytica (E. histolytica), an amebic dysentery agent, cultured in Robinson medium were investigated.
METHODS: E. histolytica cysts and trophozoites isolated from a patient with amebiasis were cultivated in the medium, incubated at 37 °C for a period of 4 days and 40 × 104/ml amebic cysts were then transferred to a fresh medium. At the second stage, 0.05, 0.1 and 0.2 mM of selected metal ions were added to the medium, and the effects of these ions on parasitic reproduction compared with the control group were observed.
RESULTS: It was determined that the number of living parasites in all the groups containing metal ions decreased significantly starting from 30 minutes (P < 0.01). CuCl2 showed the highest lethal effect on E. histolytica cysts, whereas the lowest lethal effect was observed with CoCl2. It was also seen that the number of living cells was decreased as the ion concentration and exposure time were increased, and that there were no living parasites in the medium at the end of 24 h (P < 0.01).
CONCLUSION: It may be stated that the effect of ever-increasing contamination of the environment with metal waste materials on parasites should be investigated further.
- Citation: Aksoy U, Ustun S, Dagci H, Yazar S. Effects of Cd+2, Cu+2, Ba+2 and Co+2 ions on Entamoeba histolytica cysts. World J Gastroenterol 2004; 10(3): 449-451
- URL: https://www.wjgnet.com/1007-9327/full/v10/i3/449.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i3.449
Amebiasis, which infects nearly 10% of the world population and is responsible for mortality and morbidity in developing countries in particular is caused by a protozoon, Entamoeba histolytica (E. histolytica)[1]. The form of the agent infecting humans is the 4 nuclei cysts, shed into the environment with feces. The cysts, which can survive in the environment for a long time, can carry on their life cycle with fecal-oral contamination[2,3].
Like many other protozoa, this particular parasite develops complex metabolic structures under different environmental conditions[4]. Reproduction of E. histolytica in in vitro culture provides a better understanding of its biological characteristics, its pathogenesis and adaptation to environmental conditions[2]. The metabolism of the parasite in a medium environment varies according to the age of the culture, its oxidation-reduction potential, as well as the composition, the temperature of the medium and the accompanying bacteria[5,6]. It was reported that E. histolytica cysts reproduced under axenic conditions had cyst walls, which were different from, and weaker than those found under natural conditions[7]. For this reason, it is believed that it would be more appropriate to use xenic media as they reflect the morphological structure and living conditions of the parasite[8]. One of the most commonly and successfully used xenic media is the one defined by Robinson[9].
In these complex biochemical reactions of microorganisms, certain metal cations play an important role as trace elements[10]. However, some of them are not favored by the ecosystems of living cells, and doses which accumulate in the cell in time (metal ions) do not agree with life[11,12]. Heavy metal ions in particular bind to sulphydril (-SH) groups and form complexes, leading to toxic effects on living cells. This situation can be determined by the blurring of the reproduction media of microorganisms especially in the experimental studies carried out with in vitro cultures[13]. Setting out from this point, knowing the reaction of E. histolytica cysts shed into the environment with feces to the metal ions found in the area for various reasons will especially help understand the impact of the contamination by these ions on the parasite population[14,15].
In this study, we investigated the effects of cadmium (Cd+2), copper (Cu+2), barium (Ba+2) and cobalt (Co+2) ions on E. histolytica clinical isolate xenically produced in Robinson medium. In the light of the data we obtained, we also investigated what kinds of effects these metal ions produced with different intracellular concentrations in the parasite depended on time, and whether there were any differences among these ions with respect to their tendency to produce such effects.
E. histolytica fresh clinical isolate was obtained from a patient with amebiasis who came to the laboratory of Dokuz Eylul University Medical Faculty Department of Parasitology. As a result of an examination of the stool samples of the patient by wet mount and Lugol’s iodine and trichrome staining methods, E. histolytica cysts and trophozoites were determined and the specimen was cultivated in Robinson medium[9]. The tubes were incubated at 37 °C for 4 days without subculturing. The cysts were then washed with sterile distilled water at pH 7 and counted with a haemocytometer. The 40 × 104/ml amebae cysts were transferred to a fresh medium under the same conditions and ions prepared at different molarites were immidiately added to the tubes.
Cd+2 [Cadmium chloride (CdCl2.5/2H2O) (Merck; NJ, USA)], Cu+2[Cupric chloride (CuCl2.2H2O) (Merck; NJ, USA)], Ba+2 [Bariumchloride (BaCl2.2H2O) (Riedel-de Haën; Seelze, Germany)] and Co+2 [Cobalt chloride (CoCl2.6H2O) (Carlo Erba Reagenti; Rodano (Mi), Italy)] ions prepared at 3 different molarities, namely 0.05, 0.1 and 0.2 mM, were added to the study group[15]. The remaining tubes, on the other hand, were kept as control group without addition of metal ions, and also serum was added to the control tubes. Three tubes were prepared for each ion molarity and also for control group. Each determination was performed in triplicate.
All the medium tubes were incubated at 37 °C. After 0.5, 1, 2, 3, 5, 7 and 24 h, the tubes were stirred for 5-10 seconds to achieve a homogeneous distribution and a drop of medium specimen was taken with the help of a Pasteur pipet. A drop of 1% eosin saline solution was added to the specimen. Unstained cystic structures were considered to be alive, whereas those which let pink-red stains in were regarded to be dead. Living cysts were counted with a haemocytometer and the measurements were recorded.
All the groups in this study were evaluated by means of GLM-repeated measures, post hoc Tukey in the SPSS for windows 8.0 statistical program and Mann-Whitney U test within themselves. P < 0.05 was considered significant.
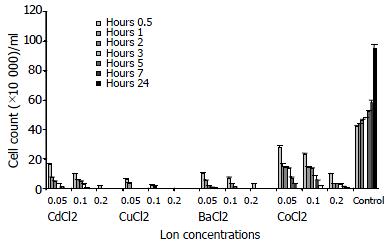
The number of living amebae per ml determined in each tube at 0.5, 1, 2, 3, 5, 7 and 24 h after metal ions and serum were added to the medium environment, is shown in Figure 1. The numbers of living cells were significantly decreased in all the groups compared to control group (P < 0.01). It was determined that the most effective metal ion was Cu+2 (P < 0.01), in contrast the least effective metal ion was Co+2. In all the groups, the number of living cells was decreased as the concentration of the metal ions was increased (P < 0.01).
Judging from the facts that they let 1% eosin-saline solution in themselves and that their cell walls degenerated and degranulated, it was found that the amebae cysts were dead after 24 h at each of the 3 concentrations of all the metal ions. It was also observed that heavy metals with larger atomic numbers affected the functions of intracellular physiologic cations, forming metal complexes. Such a complex formed with Cu+2 was seen at any concentration for Cd+2, however, the formation of complexes was started from 0.1 mM. The formation of this complex was indicated by the blurring of the medium.
As for the effect of Co+2, it was found that E. histolytica cysts survived until the end of the 7th h at 0.05 and 0.1 mM concentrations, whereas this was not the case for the other metal ions. Similarly, it was observed that at 0.2 mM of Co+2 living amebae cysts were found at the end of the first hour only (P < 0.01).
It was also observed that most of the cysts in the control group tubes, cultivated in a fresh medium, transformed into trophozoites and started to reproduce.
To be aware of the effects of toxic metals on living things is of great importance, especially in view of the ever-increasing environmental pollution. A number of researches about the effects of metal ions on the physiologic functions of various microorganisms are available[16-18].
It is believed that the toxic effect of Cd+2, an important heavy metal, might be associated with the denaturation of proteins as a result of its binding to the thiol groups in living cells or with membrane damage stemming from its interaction with Ca+2[17]. It was reported that the toxic effect of Cd+2 on the culture medium of Trypanosoma brucei brucei was at 0.045 mM level[19]. We determined in this study that when we used 0.05 mM Cd+2 as the lowest concentration, the lethal effect leading to the death of living E. histolytica cysts started at 0.5 h and was increased time-dependently. We also observed that, Cd+2 made the most complexes in the medium starting from 0.1 mM. In this context, the result we obtained was comparable with relevant literature data[19].
Cu+2, however, can interact with radicals, oxygen in particular. These radicals also cause Cu+2 to become toxic. A study carried out on pseudomonas by means of spectroscopy, reported that Cu+2 caused greater damage than Co+2 did [20]. In the present study, we discovered that the lethal effect of metal ions at different concentrations was dependent on time rather than the extent of damage and found that Cu+2 ions were more effective than Co+2. Another study in which the toxic effect of cadmium acetate and copper sulphate salts on the mouse trachea culture was investigated, reported that the effect of cadmium acetate was shown after 35 minutes, while that of copper sulphate came about after 85 minutes although equal amounts were added to the medium, accordingly the effect of cadmium acetate on the cell was greater[21]. As a different view, in a study where the cytotoxic effect of cadmium and copper was investigated in the culture of Merceneria mercenaria, the cytotoxicity concentration of Cd+2 was started at 0.1-1.5 mM and that of Cu+2 at 0.01-0.1 mM[22]. In our study, however, we investigated the effect of 0.05, 0.1 and 0.2 mM metal ions on E. histolytica cysts in a culture environment. When the effect of both metal ions was investigated starting as early as 0.5 h, we found the toxic effect of Cu+2 on amebae cysts to be greater. Cu+2 ions also bound to various compounds and formed complexes. These complexes were determined by the blurring of the medium environment and led to toxic effects on E. histolytica cysts at each molarity.
On the other hand, Co+2 enters the composition of B12, the main cofactor. This element accumulates in the core structure of microorganism cells. Ermolli et al[23] reported that 0.475 mM Co+2 produced a toxic effect on HaCaT human keratinocytes and that this effect could only be observed after 4 h. It was found in our study however, the lethal effect of Co+2 on E. histolytica was presented at lower concentrations such as 0.05 mM and was increased dose-dependently. It was also observed that amebae cysts maintained their vitality, though at a minimal level, at all the three concentrations at the end of the 7th h and that no living parasite remained in the culture medium at the end of the 24th h. In the light of these data, we believe that Co+2 at low concentrations has a slowly developing lethal effect on E. histolytica cysts.
Certain studies aiming at determining the effects of Ba+2 have been carried out in cell cultures[24,25]. Borella et al[24] reported that addition of 10(-4) -10 (-6) mol/L Ba+2 to the culture medium did not have any effect on reproduction. When we investigated the effect of 0.05, 0.1 and 0.2 mM Ba+2 on E. histolytica cysts in Robinson medium, we found that the lethal effect was dependent on both the dose and time.
CdCl2, CuCl2, BaCl2 and CoCl2 were used for evaluating the effects of these metal ions on the growth of E. histolytica cysts. As it was shown the only anion was chlor, all differed in the metal cations.
It would not be surprising to predict that investigating the effects of various factors causing environmental pollution on the life cycle of parasites, which have an important place among microorganisms, would be one of the objectives of future scientific studies. Accordingly, when the effect of Cd+2, Cu+2, Ba+2 and Co+2 ions on E. histolytica was investigated, we determined that these metal ions, led by Cu+2, prepared at concentrations of 0.05, 0.1 and 0.2 mM had a lethal effect on the parasite. Considering the fact that the life cycle of E. histolytica depends on mature cysts with 4 nuclei shed into the environment with feces, this particular result may have a considerable significance in terms of the ecological balance of the environment, which is being contaminated by various metal waste materials.
Edited by Zhu LH and Wang XL
| 1. | Walsh JA. Problems in recognition and diagnosis of amebiasis: estimation of the global magnitude of morbidity and mortality. Rev Infect Dis. 1986;8:228-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 407] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 2. | Belding DL. The parasitic amoebae of man. Textbook of Parasitology,3th Edition. Appleton-Century-Crafts, New York. 1965;31-51. |
| 3. | Vyas S, Kumar A, Piecuch S, Hidalgo G, Singh A, Anderson V, Markell MS, Baqi N. Outcome of twin pregnancy in a renal transplant recipient treated with tacrolimus. Transplantation. 1999;67:490-492. [PubMed] |
| 4. | Bruhn H, Leippe M. Novel putative saposin-like proteins of Entamoeba histolytica different from amoebapores. Biochim Biophys Acta. 2001;1514:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Clark CG, Diamond LS. Methods for cultivation of luminal parasitic protists of clinical importance. Clin Microbiol Rev. 2002;15:329-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 323] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 6. | Diamond LS. A new liquid medium for xenic cultivation of Entamoeba histolytica and other lumen-dwelling protozoa. J Parasitol. 1982;68:958-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 81] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Said-Fernández S, Mata-Cárdenas BD, González-Garza MT, Navarro-Marmolejo L, Rodríguez-Pérez E. Entamoeba histolytica cysts with a defective wall formed under axenic conditions. Parasitol Res. 1993;79:200-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Diamond LS. Axenic cultivation of Entamoeba histolytica: progress and problems. Arch Invest Med (Mex). 1980;11:47-54. [PubMed] |
| 9. | Robinson GL. The laboratory diagnosis of human parasitic amoebae. Trans R Soc Trop Med Hyg. 1968;62:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 190] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Unz RF, Shuttleworth KL. Microbial mobilization and immobilization of heavy metals. Curr Opin Biotechnol. 1996;7:307-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Agranoff DD, Krishna S. Metal ion homeostasis and intracellular parasitism. Mol Microbiol. 1998;28:403-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Vargas E, Alvarez AH, Cervantes C. [Bacterial systems for expelling toxic metals]. Rev Latinoam Microbiol. 1998;40:53-71. [PubMed] |
| 13. | Nelson N. Metal ion transporters and homeostasis. EMBO J. 1999;18:4361-4371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 223] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Campos-Góngora E, Viader-Salvadó JM, Martínez-Rodríguez HG, Zuñiga-Charles MA, Galindo JM, Said-Fernández S. Mg, Mn, and Co ions enhance the formation of Entamoeba histolytica cyst-like structures resistant to sodium dodecyl sulfate. Arch Med Res. 2000;31:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Nies DH. Microbial heavy-metal resistance. Appl Microbiol Biotechnol. 1999;51:730-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1613] [Cited by in RCA: 1176] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 16. | Kachur AV, Koch CJ, Biaglow JE. Mechanism of copper-catalyzed oxidation of glutathione. Free Radic Res. 1998;28:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 112] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Nies DH, Silver S. Ion efflux systems involved in bacterial metal resistances. J Ind Microbiol. 1995;14:186-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 266] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 18. | Weast RC. CRC handbook of chemistry and physics. 64 editors CRC, Boca Raton, Fla. 1984;. |
| 19. | Nyarko E, Hara T, Grab DJ, Tabata M, Fukuma T. Toxic effects of mercury(II), cadmium(II) and lead(II) porphyrins on Trypanosoma brucei brucei growth. Chem Biol Interact. 2002;139:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Ivanov AIu, Gavriushkin AV, Siunova TV, Khasanova LA, Khasanova ZM. [Resistance of certain strains of Pseudomonas bacteria to toxic effect of heavy metal ions]. Mikrobiologiia. 1999;68:366-374. [PubMed] |
| 21. | Olsen I, Jonsen J. Effect of cadmium acetate, copper sulphate and nickel chloride on organ cultures of mouse trachea. Acta Pharmacol Toxicol (Copenh). 1979;44:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Zaroogian G, Anderson S, Voyer RA. Individual and combined cytotoxic effects of cadmium, copper, and nickel on brown cells of Mercenaria mercenaria. Ecotoxicol Environ Saf. 1992;24:328-337. [PubMed] [DOI] [Full Text] |
| 23. | Ermolli M, Menné C, Pozzi G, Serra MA, Clerici LA. Nickel, cobalt and chromium-induced cytotoxicity and intracellular accumulation in human hacat keratinocytes. Toxicology. 2001;159:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Borella P, Manni S, Giardino A. Cadmium, nickel, chromium and lead accumulate in human lymphocytes and interfere with PHA-induced proliferation. J Trace Elem Electrolytes Health Dis. 1990;4:87-95. [PubMed] |
| 25. | Deĭneka SE, Prodanchuk NG, Petrunik IO, Davydenko IS, Sinchenko VG, Shelifost AV. [The cytotoxic action of metal stearates and its correlation with the toxicity for animals]. Gig Tr Prof Zabol. 1992;4:17-20. [PubMed] |