Published online Feb 1, 2004. doi: 10.3748/wjg.v10.i3.404
Revised: September 17, 2003
Accepted: September 24, 2003
Published online: February 1, 2004
AIM: An investigation into inflammatory bowel disease and colorectal cancer in Veszprem Province was conducted from 1977 to 2001.
METHODS: Both hospital and outpatient records were collected and reviewed comprehensively. The majority of patients were followed up regularly.
RESULTS: The population of the province was decreased from 386000 to 376000 during the period. Five hundred sixty new cases of ulcerative colitis (UC), 212 of Crohn’s disease (CD), and 40 of indeterminate colitis (IC) were diagnosed. The incidence rates increased from 1.66 to 11.01 cases per 100000 persons for UC, from 0.41 to 4.68 for CD and from 0.26 to 0.74 for IC. The prevalence rate at the end of 2001 was 142.6 for UC and 52.9 cases per 100000 persons for CD. The peak onset age in UC patients was between 30 and 40 years, in CD between 20 and 30 years. A family history of IBD was present in 3.4 % in UC and 9.9 % in CD patients. Smoking increased the risk for CD (OR = 1.94) while it decreased the risk for UC (OR = 0.25). Twelve colorectal carcinomas were observed in this cohort, the cumulative colorectal cancer risk after 10 years in UC was 2%, after 20 years 8.8%, after 30 years 13.3%.
CONCLUSION: The incidence and prevalence rates of IBD have increased steadily in Veszprem Province, now equivalent to that in Western European countries. Rapid increase in incidence rates supports a probable role for environmental factors. The rate of colorectal cancers in IBD is similar to that observed in Western countries.
- Citation: Lakatos L, Mester G, Erdelyi Z, Balogh M, Szipocs I, Kamaras G, Lakatos PL. Striking elevation in incidence and prevalence of inflammatory bowel disease in a province of western Hungary between 1977-2001. World J Gastroenterol 2004; 10(3): 404-409
- URL: https://www.wjgnet.com/1007-9327/full/v10/i3/404.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i3.404
The pathogenesis of ulcerative colitis (UC) and Crohn’s disease (CD) has only been partly understood. Inflammatory bowel disease (IBD) is a multifactorial polygenic disease with probable genetic heterogeneity. Based on this hypothesis, the disease may develop in a genetically predisposed host as a consequence of disregulated immune response to environmental factors, in particular enteric antigens, resulting in continuous immune-mediated inflammation[1,2]. However, the disease phenotype may be affected by various other factors.
IBD represents an important public health problem, as it tends to afflict young people and has a protracted and relapsing clinical course, affecting education, working abilities, social life and quality of life.
Several studies have been done on the epidemiology of IBD[3,4] . The geographical incidence of IBD varies considerably, the highest incidence rates were reported in Northern and Western Europe as well as North America, whereas they were lower in Africa, South America and Asia, including China[5]. It is more common in developed, more industrialized countries, urban residency seems to be a risk factor. The incidence rate of UC varies greatly between 0.5-24.5/100000 inhabitants, that of Crohn’s disease between 0.1-11/100000 inhabitants worldwide.
Previous studies in Europe suggested that the incidence was decreased from North to South[6,7], but in the early nineties the European IBD Study Group found comparable rates in Southern and Northern Europe[8]. This tendency may be explained by the relative stable incidence in previous high incidence areas, whereas in previous low incidence areas the incidence rose continuously. A further difference is that the previously reported predominance of UC is diminishing, as CD is becoming more prevalent. The reported average incidence of UC in Europe was 10.4, that of CD was 5.6.
Few data have been available about the frequency of IBD in the Eastern European countries. In the early eighties, Vucelic et al[9,10] conducted a prospective survey on the incidence of IBD in Zagreb, Croatia. They reported an incidence rate of 1.5/100000 inhabitants in UC and 0.7 in CD. Similarly low incidence of UC (1.7) and CD (1.4) was reported in another prospective study in Estonia[11]. Nagy et al[12] reported epidemiological data in IBD from the early 60’s to the late 80’s among Hungarians. The reported incidence rate was 3.6 in UC and 1.0 in CD. These data were based mainly on hospital reports. No population based survey has been reported.
The elevated colorectal cancer (CRC) risk in patients with long standing IBD has been widely accepted since Crohn’s original report. Recent observations suggested that the prevalence of CRC was also higher in CD[13]. The magnitude of the risk complicating IBD varied greatly in different geographic areas and in different studies. From Eastern Europe we found only one publication about CRC in IBD[14].
Recently we reported on the prevalence of extraintestinal manifestations in a large IBD cohort in a long-term follow-up study[15]. The aim of the present study was to determine the incidence and prevalence of IBD and the main epidemiological features of the disease in a province of Hungary in a population based survey.
Veszprem Province is located in the Western part of Hungary. The province consists of both industrial and agricultural regions.
The number of permanent population was relatively stable, with a slight decrease from 386462 to 376211 from 1980 to 1998 (Table 1). The rate of Gypsies is below the Hungarian average (2.5%), few Jewish people live in the province. The ratio of urban/rural residence was also relative stable (the data in 1991 were used for comparison, Table 2).
| Years | Total | Women | Men |
| 1980 | 386.462 | 194.855 | 191.607 |
| 1990 | 379.246 | 192.867 | 186.462 |
| 1998 | 376.211 | 192.296 | 183.915 |
| Urban (n) | Rural (n) | Total (n) | |
| Veszprem Province | 208.284 | 173.089 | 381.373 |
| Ulcerative colitis | 339 | 221 | 560 |
| OR: 1.27 | |||
| (95% CI: 1.07 - 1.52) | |||
| Crohn’s disease | 122 | 90 | 212 |
| OR: 1.13 | |||
| (95% CI: 0.85 - 1.49) |
There are 7 general hospitals in the province, each is staffed by at least one gastroenterologist or internist with special interest in gastroenterology. The majority of the patients (74% of UC patients and 94% of CD patients) were followed-up in the Csolnoky F. Province Hospital in Veszprem. The main data sources were the hospital records, outpatient clinical reports, endoscopical, radiological and pathological reports collected from the Internal Medicine Department, Surgery Department, Outpatient Units and family doctors. Both inpatients and outpatients permanently residing in the investigated area were included in the study. Most of the patients were followed up regularly. Diagnoses (based on hospitalization records, outpatient visits, endoscopic, radiological and histological evidence) generated in each hospital and outpatient unit were reviewed thoroughly, using the Lennard-Jones criteria[16].
Cases having another readily identifiable cause of colitis, such as infectious colitis (including pseudomembranous) were excluded. Anticoncipient or NSAID associated colitis and ischemic colitis cases were also excluded. UC cases with involvement outside the colon, with the exception of “backwash ileitis”, were excluded. In some cases the final diagnosis was made years after the beginning of symptoms.
The term indeterminate colitis (IC) was first described by Price in 1978[17] for cases operated on because of non-differentiable fulminating colitis. Today it has been used for IBD colitis cases, when the data were insufficient to differentiate between UC and CD[8].
Before the early eighties the diagnosis was based mainly on rectoscopy, histology, barium enema and upper GI series. Later colonoscopy, double-contrast barium enema and selective enterography were the basic diagnostic methods, CT and in some cases leukocyte scintigraphy were also more frequently performed to help make a more accurate diagnosis (e.g. location, activity and complications).
The location in UC was determined by colonoscopy and/or double-contrast colonography based on the macroscopic picture. Patients were classified according to the greatest known extent. In mild to moderately severe active UC cases the location was determined during the active phase, in more severe disease shortly after the active period.
The study was retrospective. Data of IBD patients were summarized yearly, in some cases the diagnosis was changed after re-evaluation. IBD patient data were collected every year from the 7 general hospitals and gastroenterology outpatient units. The provincial IBD register data were centralized in Veszprem, which is the secondary referral center for IBD patients in the province. Patients diagnosed in the same calendar year were included in the incidence calculations. All the residents and IBD patients permanently residing in the province on the 31st of December 1991 and 2001 were included in the prevalence calculations (including patients who had moved to Veszprem Province after diagnosis).
Regular colonoscopic dysplasia-cancer surveillance program with multiple biopsies was carried out in patients with extensive colitis after 8 years, with left-sided colitis after 12 years.
The source of demographic data was the Hungarian Central Statistical Office (KSH).
For statistical comparison of the data, Statistica 6.0 (Statsoft Inc., USA) was used. Odds ratio (OR) was calculated for potential factors that influenced the prevalence of IBD, including residence (urban versus rural) and smoking habits. For comparisons within group ANOVA analysis with Scheffe post hoc test was used. Yates-corrected Chi-square analysis was performed to compare differences in incidence according to the location of UC or CD during the observed period. Results were expressed as mean ± SD if otherwise not stated (NS = not statistically significant).
During the observation period 560 new patients with UC (M/F: 288/272, ratio: 1.058) and 212 new patients with CD (M/F: 108/104, ratio: 1.038) were diagnosed in Veszprem Province.
The mean incidence rate for UC was 5.89 (95% CI: 2.15 - 9.63) cases per 100000 persons per year. The sex-standardized incidence appeared slightly higher in men (6.19, 95% CI: 2.30 - 10.08) than in women (5.64, 95% CI: 2.39 - 8.89), however, the difference was not statistically significant. For the calculation of the sex standardized incidence rates, gender specific population data of Veszprem Province were used (Hungarian Central Statistical Office, KSH). In CD the average 25-year incidence was 2.23 (95% CI: 0.5 - 3.96), in men: 2.31 (95% CI: 0.64 - 3.98 and in women: 2.17 (95% CI: 0.34 - 4). Indeterminate colitis was diagnosed in forty cases (M/F: 22/18), the mean incidence rate was 0.42 cases per 100000 persons per year.
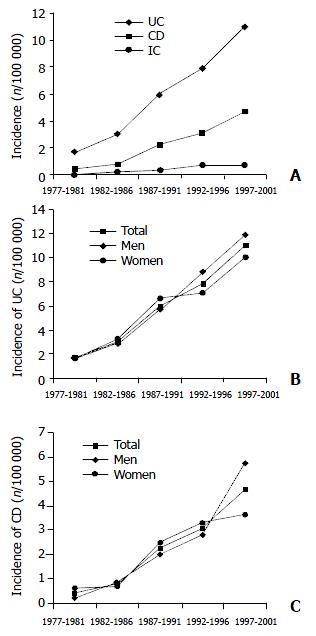
The incidence of IBD in 5-year intervals is shown in Figure 1. A sharp increase in incidence of UC was observed from 1.66 during 1977-1981 to 11.01 during 1997-2001 (in men from 1.77 to 11.96 and in women from 1.54 to 10.09). For CD, a similar tendency was observed. The incidence rose from 0.41 to 4.68, in men from 0.21 to 5.76, in women from 0.41 to 3.64. An almost continuous rise in incidence was observed for both UC and CD. The incidence of IC also rose during the observed period (from 0 to 0.74).
In contrast to UC, for which the highest incidence rate recorded was in 2001 in both sexes (in men 16.85, in women 11.96), for CD the peak incidence recorded in men was 9.24 in 1998, whereas in women it was 5.72 in 2001.
The ratio of UC/CD incidence rates decreased from 4.05 to 2. 35 during the observed periods.
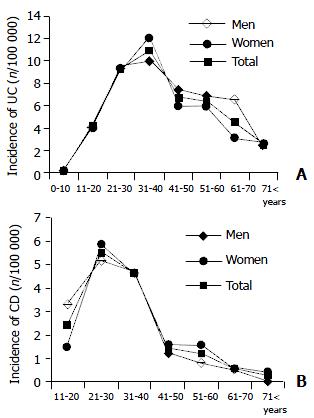
The mean age at diagnosis was 31.7 ± 12.8 years for CD, 38.9 ± 15.5 years for UC and 36.8 ± 13.9 years for IC. For the calculation of age standardized incidence rates, age specific population data of Veszprem Province were used (Hungarian Central Statistical Office, KSH). We observed only one peak incidence. For UC the highest incidence rate was in 31-40-year olds, but the 21-30-year olds incidence rate was in the same range (10.96 vs 9.26). For CD the peak incidence was in the 21-30-year olds (Figures 2A and B). The youngest UC patient was diagnosed at the age of 9 years, while the oldest patient just passed 80 years at the time of diagnosis. The youngest CD-diagnosed-patient was 12, the oldest 80 years old.
All the patients permanently living in Veszprem Province were included in the prevalence calculation (even patients diagnosed before 1977, 33 UC cases and 3 CD cases were not included as incidence cases).
On the 31st of December 1991, the prevalence of UC was 59.2 cases per 100000 persons, that of CD was 17.1 cases per 100000 persons. On this day the prevalence of IC was 2.9/100000. The prevalences were significantly increased by the 31st of December 2001. For UC the prevalence was 142.6, while for CD 52.9 cases per 100000 persons.
The urban/rural ratio was 1.205 in general population in 1991, which was relatively stable during the observed period. Urban residency seemed to increase the risk of UC (OR: 1.27), but it did not affect significantly the risk for CD (Table 2).
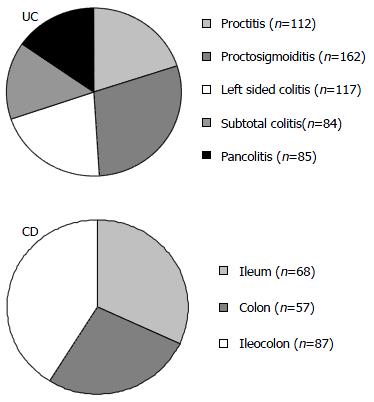
According to our information 112 of the UC patients had proctitis, 162 proctosigmoiditis, 117 left sided colitis, 84 subtotal and 85 pancolitis (Figure 3A). The age at diagnosis did not affect the location of the disease.
Location of UC did change in the past 25 years, proctitis became more prevalent in the last decade compared to the first 10 years (8.6% vs 24.0%, P = 0.002), while pancolitis cases.
were relatively less frequent (39.8% vs 27.8%, P = 0.034). The percentage of left sided colitis did not change (51.6% vs 48.2%).
The Vienna classification[18] defines CD patients according to the age at onset, location and disease behavior. In CD ileal disease (L1) was found in 32.1%, colonic (L2) in 26.9% and ileocolonic (L3) in 41.0% of the patients (Figure 3B). Location of CD did also change in the observed period. There was a tendency of colonic location becoming more frequent in the last decade compared to the first one, while the percentage of ileocolonic cases was decreased (L1: 19.2% vs 24.1%, P = NS, L2: 19.2% vs 36.5%, P = NS, L3: 61.5% vs 39.4%, P = 0.05). The most commonly associated location was perianal (48 cases). All the patients with upper gastrointestinal location (L4) had lower gastrointestinal involvement, and were classified according to this location (L1-3). In 5 cases the jejunum, in 2 the duodenum, in 5 the stomach and in 2 the esophagus were also affected. Only histologically proven cases were included in the calculation of associated locations. There was no correlation between the location and age at diagnosis.
According to the disease behavior 63 of our CD patients were defined as non-stricturing, non-penetrating (B1), 54 as stricturing (B2) and 95 as penetrating (B3). Fifty-three patients of the 95 penetrating cases had parallel strictures.
In UC group 6.4% of the patients had at least one bowel operation, a much higher frequency was observed in CD (60%, Table 3). There was an association between disease location and the frequency of operations (L1 = 0.98, L2 = 0.21, L3 = 1.93 operations on average, P < 0.01 among all the groups, ANOVA-Scheffe post hoc test).
| Surgery in UC | Surgery in CD | |
| ·Resection: 8 | Not operated: 86 (40.6 %) | |
| ·Proctocolectomy: 28 | Operated: 126 (59.4 %) | |
| - J Pouch: 25 | ·1 operation: 63 (29.7 %) | |
| ·2-3 operations: 50 (23.6 %) | ||
| ·4-8 operations: 13 (6.1 %) | ||
Of the UC patients 19 had one or more close relatives with confirmed UC or CD (Table 4). In CD patients IBD was apparent in 9.9% of the families. The occurrence of IBD was more pronounced in families of CD patients with stricturing disease than in those of CD cases with non-stricturing, non-penetrating disease (B1: 6.6% (4/60), B2: 24.5% (13/53) and B3: 11.7% (11/94), P = 0.04).
| Relationship | UC (n = 560) | CD (n = 212) |
| First-degree relative with IBD | 17 | 15 |
| Second-degree relative with IBD | 2 | 6 |
| Familial IBD | 3.4 % | 9.9% |
Sixty-seven UC patients (14.3%) were smokers at the time of diagnosis, while the rate of ex-smokers and nonsmokers was 18.4% and 67.3%, respectively (Table 5). In CD patients 102 were smokers at the time of diagnosis (50.5%, which was higher than the Hungarian average of 40.5%, 1991 data, KSH). Among the patients with CD, 13 ex-smokers and 87 nonsmokers were identified. Smoking decreased the risk for UC by 75%, while it almost doubled the risk for CD (OR: 1.94, 95% CI: 1.46 - 2.59).
| UC n (%) | CD n (%) | |
| Non-smoker | 315 (67.3%) | 87 (43.1%) |
| Ex-smoker | 86 (18.4%) | 13 (6.4%) |
| Smoker | 67 (14.3%) | 102 (50.5%) |
| No data | 92 | 10 |
| Odds ratio for smoking | 0.25 (95% CI: 0.19 - 0.32) | 1.94 (95% CI: 1.46 - 2.59) |
Colon tumor was observed in 10 patients with UC (Table 6). The average duration of IBD at the diagnosis of colorectal cancer was 18.4 years. The average age at the diagnosis of CRC was 53.4 (27 - 65) years, 15 years younger than the age of patients with non-IBD CRC at diagnosis (68.2 years, 2000 data, KSH). The male/female ratio was one (5/5). Seven out of the ten patients had pancolitis. Two colorectal cancers occurred in CD patients with a short follow-up (3 and 6 years) period. The location of CD was colonic without ileal involvement. No family history of IBD was apparent in IBD cases with CRC. Only one out of the twelve patients with IBD and CRC had a family history of CRC.
| Carcinomas | Age at diagnosis | Duration of IBD | Location of IBD | Location of tumor |
| UC (n = 10) | 35 yrs | 18.4 yrs | Pancolitis: 7 | Ascending: 2 |
| (20-56 yrs) | (9-33 yrs) | Left sided | Transverse: 4 | |
| colitis: 3 | Descending: 2 | |||
| Rectum: 2 | ||||
| CD (n = 2) | 24 and 45 yrs | 3 and 6 yrs | Right colon: 2 | Transverse: 2 |
The cumulative risk for colorectal cancer in patients with UC for 10 years was 2%, for 20 years 8.8%, and for 30 years 13.3%, respectively.
The incidence of IBD varied greatly worldwide. Genetic and environmental factors were assumed to play a significant role in the etiology of the disease[1,2]. The role of genetic factors was supported by ethnical and familial differences and also by twin studies[19-22], while the differences in incidence rates among various geographical areas suggest a role for certain environmental factors. There has been an important change in incidence of IBD in the last few decades. In high incidence countries in Western Europe the incidence rate remained relatively stable, while in previous low incidence areas[9,12] as supported by our data as well, the disease has become more common.
The continuous increase in incidence rates of both UC and CD observed in this study raises further questions. What could be the cause of this change In the first years the lower incidence rates could be explained partly by the use of less up-to-date diagnostic procedures (e.g. the relative low availability of selective enterography or colonoscopy). It is possible that better awareness, either by physicians or by patients, may result in the diagnosis of mild cases that previously might have gone unnoticed. There has also been an important change in patients’ behavior in Hungary, as patients tend to seek medical advice more often and with milder symptoms than they did two decades ago. We believe that the increase in the incidence of IBD in the second part of the observed period is real and not solely due to an improved diagnosis or more extensive search. This idea is also supported by the increase in severe cases that could only be interpreted as real. Our incidence rates in UC (11.01) and CD (4.68) in the last five-year period (1997 - 2001) were in the range as previously observed in high incidence Nordic countries[7,8,23,24] and they were much higher than the rates reported in Hungary two decades ago[12].
Most studies reported the peak onset of both UC and CD at late puberty - early adulthood period. In some studies a second peak of onset was observed in 50-70 year olds[25]. The EC-IBD study[8] reported one peak onset in CD in both sexes around the age of 20 (incidence rate in men was around 6.0 and in women 7.7) followed by a continuous decrease. In UC the peak incidence was observed in both sexes in 25-30-year olds (incidence rate was 11.2 in men and 10.7 in women), followed by a continuous decrease in women, while the incidence remained relatively stable in men. In the present study we observed one peak incidence in UC in 30-40 year olds (in men 10.0 and in women 12.1) and also in CD in 20-30 year olds (in men 5.2 and in women 5.9).
IBD could affect both sexes almost equally, with a slight predominance of women in CD, and a male/female ratio ranging from 1.1 to 2.0 in UC[5,8,23]. In our present study the male to female ratio was almost similar with a slight male predominance.
In UC the proportion of extensive colitis was higher, while the percentage of proctitis cases was lower than those in the recent EC-IBD study[8]. Location of UC cases only mildly changed in the observed period. The percentage of pancolitis cases was decreased, in contrast, procitis became more prevalent. However it was still less frequent than observed in the Western-European epidemiological studies[8].
In CD ileal disease was found in one third of the patients, and ileocolonic in 41.0% of the patients. A recent study reported a higher frequency of ileocolonic disease while the percentage of ileal disease was lower[8]. In the current study colonic involvement was observed in almost two-thirds of the patients, and there seemed to be a shift from ileal to colonic location during the observed period.
The rapid increase in incidence rates might support a role for possible environmental factors[26]. Diet, as a luminar antigen was thought to be an important factor in the pathogenesis of IBD[1,27]. In the last two decades there has been a change in the lifestyle in Hungary, as the standard way of living including the diet became more “Western”. Other possible environmental factors, such as perinatal events, infections in childhood or measles have not been investigated in this study[26,28,29]. Measles vaccination is universal in Hungary, and the disease is very rare. The birth rate is one of the lowest ones in Europe. Early childhood hygiene is developed, supporting the “oversheltered child” theory[30].
One of the most important environmental factors considered in the etiology of IBD was smoking[1,31]. In concordance with previous data we could identify smoking as a protective factor in patients with UC (OR: 0.25). In contrast, smoking increased the risk for CD almost 2-fold.
The percentage of operations in UC in our study was below the 10% - 20% rate reported in most series. The reason for this could be the more conservative practice in Hungary and also the greater resistance of patients to surgery. The majority of the operations were proctocolectomies with ileal pouch anal anastomosis (IPAA). The proportion of surgically treated patients in CD was similar to that previously published (60% - 80%). Almost one third of the CD patients had multiple operations.
There is an increased risk of CRC in IBD, but the reported incidence rates varied greatly. Earlier studies from tertiary referral centers reported rather high cumulative incidence rates of CRC (> 10% at 20 years, > 30% at 30 years). However, it might reflect referral biases, because of overrepresentation of more serious diseases in these centers that did not represent the usual spectrum of IBD[32]. In population-based studies, that followed a larger number of patients for a long time, in a certain area, lower CRC rates were reported (5.5% at 20 years, 13.5% at 30 years). The rate of colorectal cancers in our study was similar to that reported in previous population-based studies.
The two most important risk factors were the disease duration and extent (in CD the colonic involvement)[32,33]. Other factors involved are early age at onset, pharmacotherapy, disease activity (relapsing-chronically active, number and severity of relapses), smoking habits, family history of CRC, the presence of primary sclerosing cholangitis, backwash ileitis. In our study the UC patients with CRC were non-smokers while the two CD patients were smokers during the follow-up, however our numbers were relatively small to make a reasonable conclusion.
Prophylactic colectomy, regular mesalamine and folic acid treatment, and colonoscopic surveillance might reduce the elevated CRC risk[34,35]. In our study all the patients with IBD and CRC received 5-ASA. In concordance with previous results CRC was diagnosed in only one out of 32 patients with UC and in none of 66 patients with CD taking azathioprine, such azathioprine did not seem to increase cancer risk. Colonoscopic surveillance was the most widely used method to reduce CRC risk, but evidences of its benefits were controversial[36,37]. We also performed colonoscopic surveillance, which might have reduced cancer risk.
There is an additional increased risk of both colorectal and hepatobiliary malignancies in the subset of patients with primary sclerosing cholangitis (PSC) and IBD[38]. In our cohort three colorectal carcinomas developed in the 13 PSC patients, showing an increased risk in this subgroup. The reason for the short interval (3 and 6 years) between the diagnosis of CD and colorectal cancer is not clear. One may hypothesize that the asymptomatic course of IBD may be responsible for the early occurrence of colorectal cancers.
In conclusion, the incidence and prevalence have been steadily rising in the last 25 years and Now it reaches the levels reported in Western European countries. We observed no gender differences. There was a relative high incidence of colorectal cancers in IBD, similar to Western countries. The cause of the continuous rapid increase in the incidence of IBD is unknown, but the incidence supports a possible role for environmental (e.g. diet, lifestyle) factors.
The authors thank the following doctors for their help in data collection: Gyula David, M.D., Agnes Horvath, M.D. and Tunde Pandur, M.D. (Veszprem), Sandor Meszaros MD, Pal Küronya, M.D. and Csaba Molnar, M.D. (Ajka), Zsuzsa Balogh, M.D. (Papa) and Arpad Tollas, M.D. (Varpalota), and Mrs. Gabriella Deményi for her technical assistance.
| 1. | Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2693] [Cited by in RCA: 2773] [Article Influence: 115.5] [Reference Citation Analysis (3)] |
| 2. | Tibble JA, Bjarnason I. Non-invasive investigation of inflammatory bowel disease. World J Gastroenterol. 2001;7:460-465. [PubMed] |
| 3. | Delcò F, Sonnenberg A. Commonalities in the time trends of Crohn's disease and ulcerative colitis. Am J Gastroenterol. 1999;94:2171-2176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Niv Y, Abuksis G, Fraser GM. Epidemiology of Crohn's disease in Israel: a survey of Israeli kibbutz settlements. Am J Gastroenterol. 1999;94:2961-2965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Xia B, Shivananda S, Zhang GS, Yi JY, Crusius JBA, Peka AS. Inflammatory bowel disease in Hubei province of China. China Natl J New Gastroenterol. 1997;3:119-120. |
| 6. | Tsianos EV, Masalas CN, Merkouropoulos M, Dalekos GN, Logan RF. Incidence of inflammatory bowel disease in north west Greece: rarity of Crohn's disease in an area where ulcerative colitis is common. Gut. 1994;35:369-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Stewénius J, Adnerhill I, Ekelund G, Florén CH, Fork FT, Janzon L, Lindström C, Mars I, Nyman M, Rosengren JE. Ulcerative colitis and indeterminate colitis in the city of Malmö, Sweden. A 25-year incidence study. Scand J Gastroenterol. 1995;30:38-43. [PubMed] |
| 8. | Shivananda S, Lennard-Jones J, Logan R, Fear N, Price A, Carpenter L, van Blankenstein M. Incidence of inflammatory bowel disease across Europe: is there a difference between north and south Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD). Gut. 1996;39:690-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 684] [Cited by in RCA: 668] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 9. | Vucelić B, Korać B, Sentić M, Milicić D, Hadzić N, Juresa V, Bozikov J, Rotkvić I, Buljevac M, Kovacević I. Ulcerative colitis in Zagreb, Yugoslavia: incidence and prevalence 1980-1989. Int J Epidemiol. 1991;20:1043-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Vucelić B, Korać B, Sentić M, Milicić D, Hadzić N, Juresa V, Bozikov J, Rotkvić I, Buljevac M, Kovacević I. Epidemiology of Crohn's disease in Zagreb, Yugoslavia: a ten-year prospective study. Int J Epidemiol. 1991;20:216-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Salupere R. Inflammatory bowel disease in Estonia: a prospective epidemiologic study 1993-1998. World J Gastroenterol. 2001;7:387-388. [PubMed] |
| 12. | Nagy G, Minik K, Ujszaszy L, Juhasz L. Epidemiology of inflammatory bowel diseases in Borsod-Abauj-Zemplen county 1963-a) 1992. LAM. 1994;4:424-430. |
| 13. | Friedman S, Rubin PH, Bodian C, Goldstein E, Harpaz N, Present DH. Screening and surveillance colonoscopy in chronic Crohn's colitis. Gastroenterology. 2001;120:820-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 168] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 14. | Maratka Z, Nedbal J, Kociánová J, Havelka J, Kudrmann J, Hendl J. Incidence of colorectal cancer in proctocolitis: a retrospective study of 959 cases over 40 years. Gut. 1985;26:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Lakatos L, Pandur T, David G, Balogh Z, Kuronya P, Tollas A, Lakatos PL. Association of extraintestinal manifestations of inflammatory bowel disease in a province of western Hungary with disease phenotype: results of a 25-year follow-up study. World J Gastroenterol. 2003;9:2300-2307. [PubMed] |
| 16. | Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2-6; discussion 16-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1459] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 17. | Price AB. Overlap in the spectrum of non-specific inflammatory bowel disease--'colitis indeterminate'. J Clin Pathol. 1978;31:567-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 222] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J. Behaviour of Crohn's disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 684] [Cited by in RCA: 715] [Article Influence: 28.6] [Reference Citation Analysis (1)] |
| 19. | Loftus EV, Silverstein MD, Sandborn WJ, Tremaine WJ, Harmsen WS, Zinsmeister AR. Ulcerative colitis in Olmsted County, Minnesota, 1940-1993: incidence, prevalence, and survival. Gut. 2000;46:336-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 360] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 20. | Zheng CQ, Hu GZ, Zeng ZS, Lin LJ, Gu GG. Progress in searching for susceptibility gene for inflammatory bowel disease by positional cloning. World J Gastroenterol. 2003;9:1646-1656. [PubMed] |
| 21. | Kinouchi Y, Matsumoto K, Negoro K, Takagi S, Takahashi S, Hiwatashi N, Shimosegawa T. Hla-B genotype in Japanese patients with Crohn's disease. Dis Colon Rectum. 2003;46:S10-S14. [PubMed] |
| 22. | Peña AS. Genetics of inflammatory bowel diseases--past, present, and future. Dig Dis. 2003;21:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Björnsson S, Jóhannsson JH. Inflammatory bowel disease in Iceland, 1990-1994: a prospective, nationwide, epidemiological study. Eur J Gastroenterol Hepatol. 2000;12:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 85] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Lindberg E, Jörnerot G. The incidence of Crohn's disease is not decreasing in Sweden. Scand J Gastroenterol. 1991;26:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Stowe SP, Redmond SR, Stormont JM, Shah AN, Chessin LN, Segal HL, Chey WY. An epidemiologic study of inflammatory bowel disease in Rochester, New York. Hospital incidence. Gastroenterology. 1990;98:104-110. [PubMed] |
| 26. | Kugathasan S, Judd RH, Hoffmann RG, Heikenen J, Telega G, Khan F, Weisdorf-Schindele S, San Pablo W Jr, Perrault J, Park R. Epidemiologic and clinical characteristics of children with newly diagnosed inflam-matory bowel disease in Wisconsin: a statewide population-based study. J Pediatr. 2003;143:525-531. [RCA] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 406] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 27. | Cashman KD, Shanahan F. Is nutrition an aetiological factor for inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2003;15:607-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Delcò F, Sonnenberg A. Exposure to risk factors for ulcerative colitis occurs during an early period of life. Am J Gastroenterol. 1999;94:679-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Elliman DA, Bedford HE. Measles, mumps and rubella vaccine, autism and inflammatory bowel disease: advising concerned parents. Paediatr Drugs. 2002;4:631-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Gilat T, Hacohen D, Lilos P, Langman MJ. Childhood factors in ulcerative colitis and Crohn's disease. An international cooperative study. Scand J Gastroenterol. 1987;22:1009-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 236] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 31. | Picco MF, Bayless TM. Tobacco consumption and disease duration are associated with fistulizing and stricturing behaviors in the first 8 years of Crohn's disease. Am J Gastroenterol. 2003;98:363-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Munkholm P. Review article: the incidence and prevalence of colorectal cancer in inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18 Suppl 2:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 400] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 33. | Eaden JA, Mayberry JF. Colorectal cancer complicating ulcerative colitis: a review. Am J Gastroenterol. 2000;95:2710-2719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 75] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Shanahan F. Review article: colitis-associated cancer -- time for new strategies. Aliment Pharmacol Ther. 2003;18 Suppl 2:6-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Eaden J. Review article: the data supporting a role for aminosalicylates in the chemoprevention of colorectal cancer in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18 Suppl 2:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Hata K, Watanabe T, Kazama S, Suzuki K, Shinozaki M, Yokoyama T, Matsuda K, Muto T, Nagawa H. Earlier surveillance colonoscopy programme improves survival in patients with ulcerative colitis associated colorectal cancer: results of a 23-year surveillance programme in the Japanese population. Br J Cancer. 2003;89:1232-1236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Karlén P, Kornfeld D, Broström O, Löfberg R, Persson PG, Ekbom A. Is colonoscopic surveillance reducing colorectal cancer mortality in ulcerative colitis A population based case control study. Gut. 1998;42:711-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 195] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 38. | Shetty K, Rybicki L, Brzezinski A, Carey WD, Lashner BA. The risk for cancer or dysplasia in ulcerative colitis patients with primary sclerosing cholangitis. Am J Gastroenterol. 1999;94:1643-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 167] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
Edited by Zhu LH and Wang XL