INTRODUCTION
The activation and proliferation of hepatic stellate cells (HSC) have been regarded as the critical events in early hepatic fibrosis irrespective of the underlying etiology[1-6]. c-myb oncogene encodes nuclear protein that functions as a transcriptional transactivator that regulates expression of genes critical for cell differentiation and proliferation[7,8]. Several studies have indicated that c-myb expression is critical for HSC activation and proliferation in rat and human livers affected by chronic viral hepatitis, and that the level of c-myb expression reflects the severity of disease activity[9,10]. However, little information is available concerning the effects of c-myb expression on the pathogenesis of liver fibrosis.
It has been shown that α1-I collagen is the main extracellular matrix (ECM) protein produced by HSCs during the pathogenesis of live fibrosis, while TGF-β1 has been identified as a potent cytokine in the regulation of the production, degradation and accumulation of ECM. In this study, retrovirus-mediated antisense c-myb RNA was transferred into cultured HSCs from rats to investigate its anti-proliferative and antifibrotic effects.
MATERIALS AND METHODS
Materials
PA317 and NIH3T3 cells were cultured in our laboratory. Anti-desmin and anti-α-SMA antibodies were purchased from DAKO Corporation, anti-c-myb antibody from Santa Cruz Biotechnology Incorporation. G418, DMEM, and fetal bovin serum were from GibcoTM Invitrogen Corporation. Pronase E and collagenase IV were from Sigma-Aldrich Corporation. The retroviral vector pDOR was provided by Dr. Lin Yang (Department of Infectious Diseases, the 3rd Affiliated Hospital, Sun-Yat Sen University).
Cell isolation, culture and identification
Primary rat HSCs were isolated from 200-300 g male Sprague-Dawley rats by a two-step pronase-collagenase perfusion method[11]. All cells were cultured in DMEM supplemented with 100-200 mL/L fetal bovine serum, 2 mmol/L glutamine and 1% antibiotic solution at 37 °C in a humidified atmosphere containing 50 mL/L CO2. HSCs were identified by immunohistochemical staining for desmin and α-SMA with monoclonal mouse anti-desmin and anti-α-SMA antibodies, respectively.
Packaging of c-myb recombinant retroviral vector and its transfection into HSCs
The segment of c-myb gene amplified by RT-PCR from the spleen of a rat was cloned into pUC19 with TA cloning method, and then subcloned into retroviral vector pDOR after sequencing. The recombinant retroviral vector, named pDOR-myb, was transfected into retroviral package cell line PA317 by means of DOTAP. Stable retroviral vector- produced lines were generated by expanding the G418-resistant (600 µg/mL) colonies. The supernatants containing the packaged retroviruses were harvested, filtered and titrated 9.0 × 104-2.2 × 105 CFU/mL determined in NIH3T3 cells[12]. The fourth generation of HSCs was infected with the recombinant c-myb retrovirus (pDOR-myb) and controlled retrovirus (pDOR), while the untreated HSCs from the same generation were used as the control group. Infection was performed in suspension by a 30 min incubation of HSCs with 10 mL of virus supernatants, supplemented with 4 µg/mL polybrene. The cultured medium was changed with DMEM containing G418 after 48 h, and repeated every 2-3 d.
Proliferation assay of infected HSCs
The cell proliferation was measured by 3-[4, 5-Dimethylthiazolzyl]-2, 5-diphenyl tetrazo-dium bromide (MTT) method. HSCs of three groups (pDOR-myb, pDOR and control groups) were seeded into 96-well plates (5 × 103 cells/well), respectively and incubated in a final volume of 200 µL medium for 48 h. Fifty micriliter of MTT (5 g/L) was subsequently added to each well. After the incubation was continued for 4 h, suspension was abandoned, and dimethyl sulphoxide (DMSO) 200 µL was added to each well. Then absorbance at 570 nm (A570 nm) was measured using an automatic enzyme-linked immunosorbent assay plate reader.
Semi-quantitative reverse transeription-polymerase chain reaction (RT-PCR)
For reverse transcription PCR analysis, total RNA was extracted from cultured cells using a RNeasy Mini kit (QIAGEN). Complementary DNAs were prepared from 1 µg of total RNA using a RT kit (MBI) with oligo (dt) primer according to the manufacturer’s instructions. The resulting complementary DNA was amplified with the following sets of primers: GAPDH (420 bp), 5’ATGACTCTACCCACGGCAAG3’ (forward)and 5’CCACAGTCTTCTGAGTGGCA3’ (reverse); TGF-β1 (143 bp),5’GACCTGCTGGCAATAGCTTC3’ (forward) and 5’GTTGAGGGAGAAAGCAGCAG3’ (reverse); α1-collagen (269 bp), 5’GCGAGGACATGAGGAGTAGC3’ (forward) and 5’CCTGTGACCAGGGATGTCTT3’ (reverse); c-myb (350 bp), 5’TCCTTCTCCTCCTCCTCCTC3’ (forward) and 5’GTTCCACCAGCTTCTTAGC3’ (reverse). c-myb, TGF-β1 and α1-I collagen mRNA levels were determined by semi-quantitative RT-PCR followed by densitometry scanning. GAPDH served as an internal control. The following PCR program was performed for TGF-β1 and α1-I collagen: at 94 °C for 2 min (initial denaturation), at 94 °C for 30 s, at 62 °C for 30 s, at 72 °C for 1 min, 35 cycles. For c-myb, except the primer annealing temperature was 65.5 °C, all other conditions were the same as above.
Western-blotting
c-myb proteins of the pDOR-myb group, pDOR group and control group were detected with Western-bloting. HSCs of the three groups were washed 2 times with PBS, and then 1 × SDS loading buffer was added. After boiled for 5-10 min, 10 µg of protein was electrophoresed on 100 g/L SDS-polyacrylamide gel. The protein was transferred to PVDF membrane, which was then blocked overnight at 4 °C with PBST (PBS containing 4 mL/L Tween 20)-30 g/LBSA. The blots were incubated with the primary polyclonal antibody against c-myb (1:100) at 4 °C overnight, and subsequently with HRP-labeled secondary antibody (1:100) at room temperature for 1 h. At last, the protein bands were visualized with 4-Cl-1-Naphenol-H2O2.
Statistical analysis
Data were presented as mean ± SE. The Student’s t test was to analyze the changes in different groups with SPSS10.0 software, and P < 0.05 was considered statistically significant.
RESULTS
HSCs identification
The number of isolated cells was 1-3 × 107 from a rat with the viability > 98%. Immunohistochemical staining with desmin and α-SMA showed a selective cytoplasma pattern with about 95% positive cells.
c-myb expression
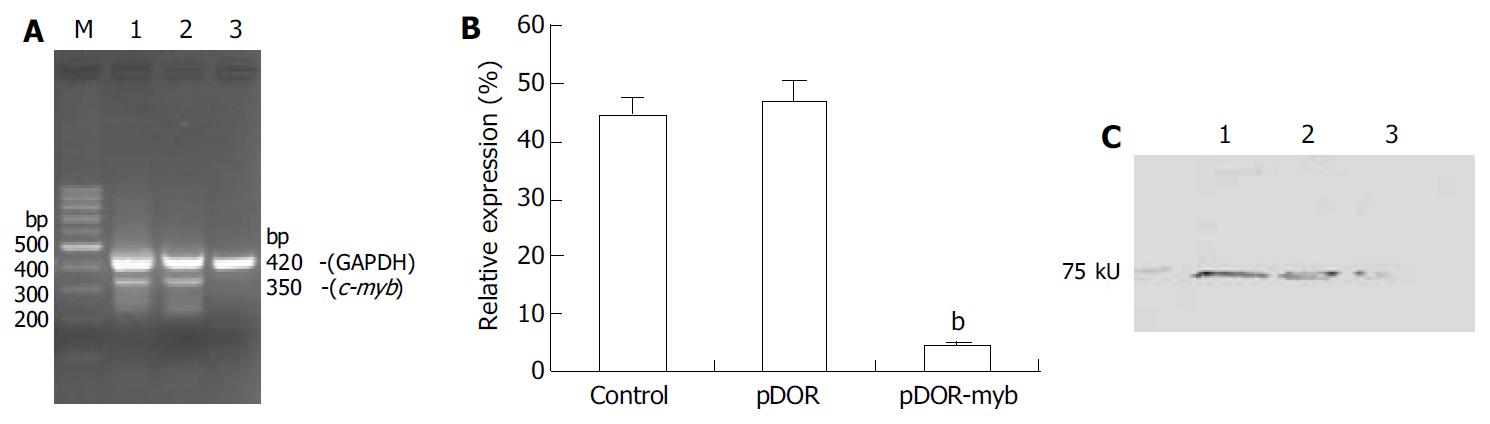
Semi-quantitative RT-PCR and Western-blot were performed to determine c-myb expression in the recombinant c-myb virus infected HSCs. RT-PCR showed a 420 bp band corresponding to GAPDH in each sample. As expected, a 350 bp band of c-myb was detected in the pDOR group and the control group, but not in the pDOR-myb group, and the results of densitometry scanning showed that c-myb mRNA expression value in the pDOR-myb group was 4.63 ± 0.66, significantly lower than that in the control (44.48 ± 2.79) and pDOR groups (46.91 ± 3.57) (P < 0.01). Western blot analysis demonstrated that c-myb protein was markedly reduced in the pDOR-myb group compared with the control group. (Figure 1).
Figure 1 Expression of c-myb mRNA, internal control and c-myb protein by RT-PCR, density and Western blot.
A: c-myb mRNA expression assayed by semi-quantitative RT-PCR analysis of total RNA. Co-amplification of GAPDH in all samples served as an internal standard. Lane 1: control HSC; Lane 2: pDOR treated HSC; Lane 3: pDOR-myb treated HSC; Lane M: 100 bp ladder. B: Relative expression levels are shown as percent of the internal control by densitometry scanning. The results were expressed as mean ± SE (n = 3). bP < 0.01 vs pDOR and control. C: Western-blotting of c-myb protein expression. Fifty µg of extracted cellular protein was loaded in each lane. Lane1: control HSC; Lane2: pDOR treated HSC; Lane3: pDOR-myb treated HSC.
HSC proliferation
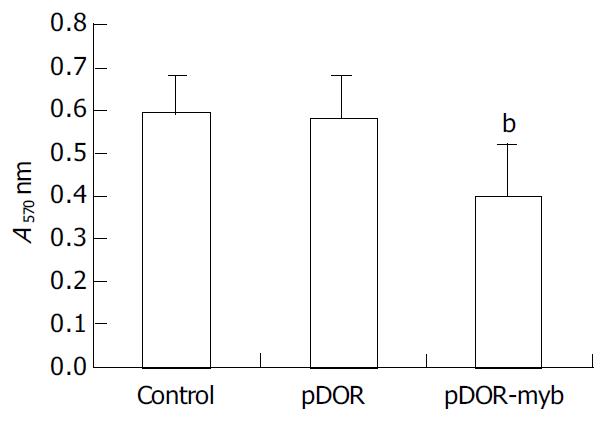
Proliferation assay showed that A570 nm values of the pDOR-myb group, pDOR group and control group were 0.40 ± 0.12, 0.58 ± 0.10 and 0.59 ± 0.09, respectively, indicating a marked decrease in the pDOR-myb group compared with the control groups (P < 0.01), but no difference between two control groups (Figure 2).
Figure 2 Measurements of cell proliferation with MTT method in three groups of HSCs.
The results were expressed as mean ± SE (n = 6). bP < 0.01 vs pDOR and control.
TGF-β1 and α1-I collagen mRNA expression
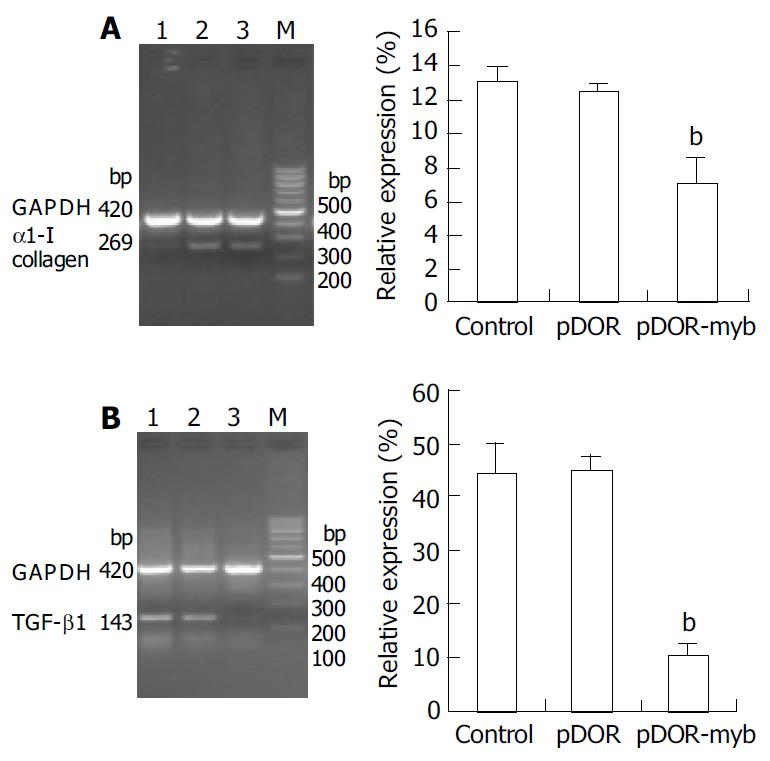
Semi-quantitative RT-PCR was used to analyze TGF-β1 and α1-I collagen mRNA expression. The mRNA levels of α1-I collagen and TGF-β1 were markedly reduced in the pDOR-myb group compared with the two control groups (P < 0.01). The results of densitometry scanning showed that α1-I collagen and TGF-β1 mRNA expression values in the pDOR-myb group were 6.94 ± 1.58 and 10.09 ± 2.26, respectively, which were markedly reduced compared with the control (44.10 ± 5.63 and 13.01 ± 0.94, respectively) and pDOR groups (44.59 ± 2.54 and 12.47 ± 0.48, respectively) (P < 0.01). But there was no difference between the two control groups (Figure 3).
Figure 3 Expression of TGF-β1 and α1-I collagen mRNA.
A: α1-I collagen mRNA expression assayed by semi-quantitative RT-PCR analysis. Lane 1: pDOR-myb treated HSC; Lane 2: control HSC; Lane 3: pDOR treated HSC. B: TGF-β1 mRNA expression assayed by semi-quantitative RT-PCR analysis. Lane 1: pDOR treated HSC; Lane 2: control HSC; Lane 3: pDOR-myb treated HSC. The relative expression levels were shown as percent of the internal control by densitometry scanning. The results were expressed as mean ± SE (n = 3), bP < 0.01 vs pDOR and control. Co-amplification of GAPDH in all samples served as an inter-nal standard.
DISCUSSION
HSC is regarded as one of the key cell types involved in progression of liver fibrosis, and as a therapeutic target for treatment of hepatic fibrosis[1-6]. Several findings have implied that HSC activation status could be directly manipulated through controlled expression of c-myb. c-myb was reported to be involved in transcriptional regulation and cell proliferation, since high levels of c-myb DNA-binding activity were found in activated HSCs but not in quiescent HSCs[13,14]. Transfection of c-myb stimulated α-SMA expression in quiescent HSCs, and transfection of c-myb antisense RNA inhibited the expression of α-SMA and the activation of HSCs[14].
In this study, we found that the c-myb expression in HSCs could be successfully inhibited by c-myb antisense RNA transfection and this antisense c-myb RNA could dramatically inhibit HSC proliferation detected by MTT, suggesting that blockade of c-myb transcriptional signals in HSC might lead to the inhibition of HSC proliferation. However, little is known about how c-myb expression can influence HSC activation and proliferation. It has been reported that c-myb is an important transcriptional factor that can regulate cell cycles and control plasma membrane Ca2+- ATPase[15,16], which may be involved in HSC proliferation.
TGF-β1 is the most potent fibrogenic stimulant to HSCs. The activated HSCs respond to it by increasing production of collagen and decreasing its breakdown. Modifying the secretion or activity of TGF-β1 could attenuate fibrosis, and recent studies of experimental liver fibrosis have shown the potential of this approach[17-22]. Our study showed that TGF-β1 mRNA expression was repressed significantly in the pDOR-c-myb infected group compared with their corresponding control groups. The mechanism might be partly related to the fact that c-myb antisense RNA could inhibit HSC proliferation and block TGF-β1 autocrine loop. It is necessary to investigate HSC activation and its relationship with c-myb expression and TGF-β1 secretion, since c-myb also plays an important role in HSCs activation[1,14].
Hepatic fibrosis is characterized by accumulation of extracellular matrix (ECM), and collagen I is the main component of ECM while fibrosis occurs[23-26]. Activated HSCs are known to be the major source of this fibrillar collagen. On the other hand, collagen I could further enhance HSC activation[1]. Clearly, this feedback loop plays an important role in the progression of liver fibrosis. In our study, α1-I collagen mRNA expression in HSCs was inhibited significantly by transfection of c-myb antisense RNA. Considering c-myb antisene RNA could also inhibit HSC proliferation, which might influence α1-I collagen mRNA expression levels, the number of HSCs in each group was adjusted to the same count. Our results indicated that c-myb could influence α1-I collagen expression through some direct but yet unclear ways.
Liver fibrosis and its end stage, cirrhosis, represent worldwide healthcare problems. Current treatments for fibrosis and cirrhosis are limited to removing the underlying injurious stimuli, eradicating viruses using interferon, ribavirin, and lamivudine in viral hepatitis, and liver transplantation. To date, various new drugs have been attempted to prevent the progression of hepatic fibrosis, such as hepatocyte growth factor[26,27], TGF-β1 antagonist or truncated TGF-β1 type II receptor[21,28,29] and tissue inhibitors of metalloproteinases[30]. Now that HSC activation and proliferation have been shown to be the critical events, and also to contribute to portal hypertension. Hence,the strategies to design some specific agents to inhibit HSC activation and proliferation are appealing. In conclusion,c-myb antisense RNA can inhibit c-myb gene expression, cell proliferation, α1-I collagen and TGF-β1 mRNA expression in HSCs, suggesting that inhibition of c-myb gene expression might be a potential way for the treatment of liver fibrosis.