Published online Dec 15, 2004. doi: 10.3748/wjg.v10.i24.3654
Revised: May 6, 2004
Accepted: May 13, 2004
Published online: December 15, 2004
AIM: To investigate the effect of pyrrolidine dithiocarbamate (PDTC), a novel nuclear factor-κB (NF-κB) inhibitor, on expression of multiple inflammatory mediators and neutrophilic inflammation of cold preserved grafts after rat liver transplantation and its significance.
METHODS: Orthotopic liver transplantation (OLT) was performed after 24 h of cold storage using University of Wisconsin solution with varied concentrations of PDTC. We determined the time course of NF-κB activation and expression of multiple inflammatory signals, such as tumor necrosis factor-α (TNF-α), cytokine-inducible neutrophil chemoattractant (CINC), and intercellular adhesion molecule-1 (ICAM-1) by ELISA methods. Serum alanine aminotransferase (ALT), intrahepatic myeloperoxidase (MPO)/WBC (a measure of neutrophil accumulation) and Mac-1 expression (a measure of circulating neutrophil activity) were also evaluated.
RESULTS: PDTC decreased NF-κB activation induced by prolonged cold preservation in a dose dependent manner (from 20 mmol/L to 60 mmol/L), diminished TNF-α, CINC, ICAM-1 proteins in the grafts, and reduced the expression of increases in plasma TNF-α levels induced by prolonged cold preservation. Neutrophilic inflammation of the graft was significantly suppressed after preservation with PDTC (P < 0.05). The total neutrophil accumulation in PDTC (40 mmol/L) group (7.04 ± 0.97) was markedly reduced compared to control group (14.07 ± 1.31) (P < 0.05). Mac-1 expression was significantly reduced in PDTC (40 mmol/L) group (181 ± 11.3%) compared with the control group (281 ± 13.2%) (P < 0.05) at 6 h after reperfusion. Furthermore, PDTC inhibited the increased serum ALT levels after liver transplantation.
CONCLUSION: PDTC can inhibit B NF-κB activation and expression of the inflammatory mediators, which are associated with improved graft viability via inhibiting intrahepatic neutrophilic inflammation. Our study suggests that a therapeutic strategy directed at inhibition of NF-κB activation in the transplanted liver might be effective in reducing intrahepatic neutrophilic inflammation, and would be beneficial to cold preserved grafts.
-
Citation: Gu XP, Qiu YD, Xu FT, Jiang Y, Ding YT.
In vivo suppressive effect of nuclear factor-κB inhibitor on neutrophilic inflammation of grafts after orthotopic liver transplantation in rats. World J Gastroenterol 2004; 10(24): 3654-3658 - URL: https://www.wjgnet.com/1007-9327/full/v10/i24/3654.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i24.3654
Poor graft viability is a frequent complication of liver transplantation, the incidence of primary graft nonfunction was reported to be 2%-23%[1]. Furukawa et al[2] reported that the primary nonfunction rate and retransplantation rate significantly rose as cold-ischemia time increased, especially beyond 20 h. It is currently accepted that sinusoidal endothelial cells (SECs) are the primary targets of cold ischemia-reperfusion injury[3], however, parenchymal cell damage has direct effects on the deterioration of liver function. The functional impairment of the grafted liver, therefore, seems to be complex and multifactorial. Polymorphonuclear leukocytes (PMNs) have been implicated as a causal factor in the development of ischemia-reperfusion injury[4,5]. In the previous study, we found the promoted neutrophil accumulation and activity in cold preserved grafts, and the evidence confirmed that cold preservation-reperfusion injury of liver allografts was a chain of cascades induced by activation of a variety of inflammatory mediators from early to late phase after reperfusion[6]. Protective strategies were confused that inhibition of one mediator was always compensated by activation of other mediators from different pathways[8]. Researchers are anxious to look for the core mediators that can trigger multiple proinflammatory genes. Recent studies have shown that transcription factor NF-κB plays an essential role in regulation of these kinds of genes whose products take part in the pathogenesis of preservation-reperfusion injury in liver allografts[6-11]. Hence, it is hypothesized that NF-κB may be an appropriate target for treatment of reperfusion injury. NF-κB appears to have both beneficial and harmful effects on liver transplantation[12-16]. It was reported that NF-κB could promote liver regeneration and prevent apoptosis[14], and contribute to the inflammatory response of ischemia/reperfusion[15]. In the present study, we determined the time course of NF-κB activation after OLT in relation to the expression of tumor necrosis factor-α (TNF-α), intercellular adhesion molecule-1 (ICAM-1), and cytokine-induced neutrophil chemoattractant (CINC). The selectivity of pyrrolidine dithiocarbamate (PDTC) as an in vivo inhibitor of NF-κB activation, the effects of PDTC on OLT-induced expression of these inflammatory gene products and the resultant hepatic neutrophilic inflammation, and the action of NF-κB were studied.
Following reagent were purchased: Pyrrolidine dithiocarbamate (PDTC) from Sigma, nuclear extract kit and NF-κB p65 Transcription factor assay kits from Active Motif, Pierce BCA protein assay reagent and MPO assay reagent from Jiancheng, Nanjing, ELISA assay kit for CINC, TNF-α and ICAM-1 from Amersham, Buckinghamshire, UK, FACS lysing solution from Becton Dickinson, San Jose, CA and FITC-labeled mouse anti-rat CD11b antibody from Camarillo, CA.
Male wistar rats, weighing 200 to 250 g, were purchased from Nanjing Medical Institute Animal Care Committee (Grade II, Certification No 2002-0017). Prior to the experiment, rats were fasted for 12 h and allowed free access to water.
Rats were allocated randomly into four groups (36 rats in each group): control group in which the grafts were harvested with injection of University of Wisconsin (UW) solution, PDTC20 group in which the grafts were harvested with the preservation solution added by 20 mmol/L PDTC, PDTC40 group in which the grafts were harvested with the preservation solution added by 40 mmol/L PDTC, and PDTC60 group in which the grafts were harvested with the preservation solution added by 60 mmol/L PDTC.
Liver harvesting and orthotopic transplantation were performed according to the methods described by Kamada and Calne[17], with some minor modifications[18], and the hepatic artery was not reconstructed. The graft liver was preserved with University of Wisconsin (UW) solution at 4 °C for 24 h. The portal vein clamping time in recipients varied from 18 to 20 min, implantation time was less than 50 min, all the procedures took about 60 min. No significant difference was seen in portal clamping time between groups. The recipients were sacrificed 0 h, 0.5 h, 1 h, 2 h, 4 h, and 6 h after reperfusion. Blood samples were collected from the hepatic vein draining the left lateral lobe as previously described to determine the serum levels of alanine aminotransferase (ALT) and neutrophil activity at indicated time points. The median lobe of the liver was carefully excised at the designated time point and stored at -80 °C for analysis.
Preparation of nuclear extracts using a nuclear extract kit and determination of p65/relA subunits of NF-κB using a TransAM NF-κB p65 kit according to the manufacturer’s instructions. The concentration of p65/relA subunits in liver tissue homogenates was standardized to the total nuclear protein content in each specimen as measured by the Pierce BCA protein assay reagent. NF-κB activity was expressed as p65/relA subunits μg/mg total nuclear protein.
Cellular function and damage were estimated by the peak serum ALT activity in six rats from each group at selected time points after reperfusion. Serum ALT was measured using the Opera clinical chemistry system.
Serum TNF-α and CINC were measured in duplicate by using the immunoassay kits according to the manufacturer’s instructions.
Hepatic levels of TNF-α, CINC, and ICAM-1 were determined as previously described[19]. In brief, the medial hepatic lobe was weighed and homogenized in 5 mL of 0.1 mol/L phosphate buffer (pH7.4) containing 0.5 g/L of sodium azide at 4 °C. The homogenates were centrifuged at 2000 r/min for 10 min to remove solid tissue debris. The supernatant was assayed using a rat ELISA system. The concentration of antigens in liver tissue homogenates was standardized to the total protein content in each specimen as measured by the Pierce BCA protein assay reagent. The results were expressed as pictograms per gram of tissue (pg/g).
The activity of MPO, an enzyme in azurophilic granules of neutrophils, was used as a well-established marker to quantitate tissue neutrophil sequestration[20]. Frozen livers were thawed and extracted for MPO, following the homogenization and sonication procedure as described in the manufacturer’s protocol. One unit of MPO activity was defined as the amount of enzyme able to reduce 1 μmol of peroxide/min. Results were expressed as units of MPO activity per gram of tissue. To control intarvascular leukocytosis, we calculated the ratio of MPO activity in tissue to WBC count in peripheral blood, and expressed it as MPO/WBC.
Blood samples collected from each animal at each time point were subjected to flow cytometric analysis[21]. For CD11b and L-selectin determination, 50 μL of samples were incubated with 10 μL of PE-labeled mouse anti-rat L-selectin antibody and 10 μL of FITC-labeled mouse anti-rat CD11b antibody for 15 min, then 1 mL of FACS lysing solution was added for 10 min, followed by centrifugation at 1500 r/min for 5 min .Cell pellets were washed and then resuspended in 500 μL of PBS. The cells were read on a Becton-Dickinson FACS calibur.
The data were expressed as mean ± SE. Statistical analysis was performed using analysis of variance in SPSS software (version 11.0 for windows), followed by SNK multiple comparisons. P less than 0.05 were considered statistically significant.
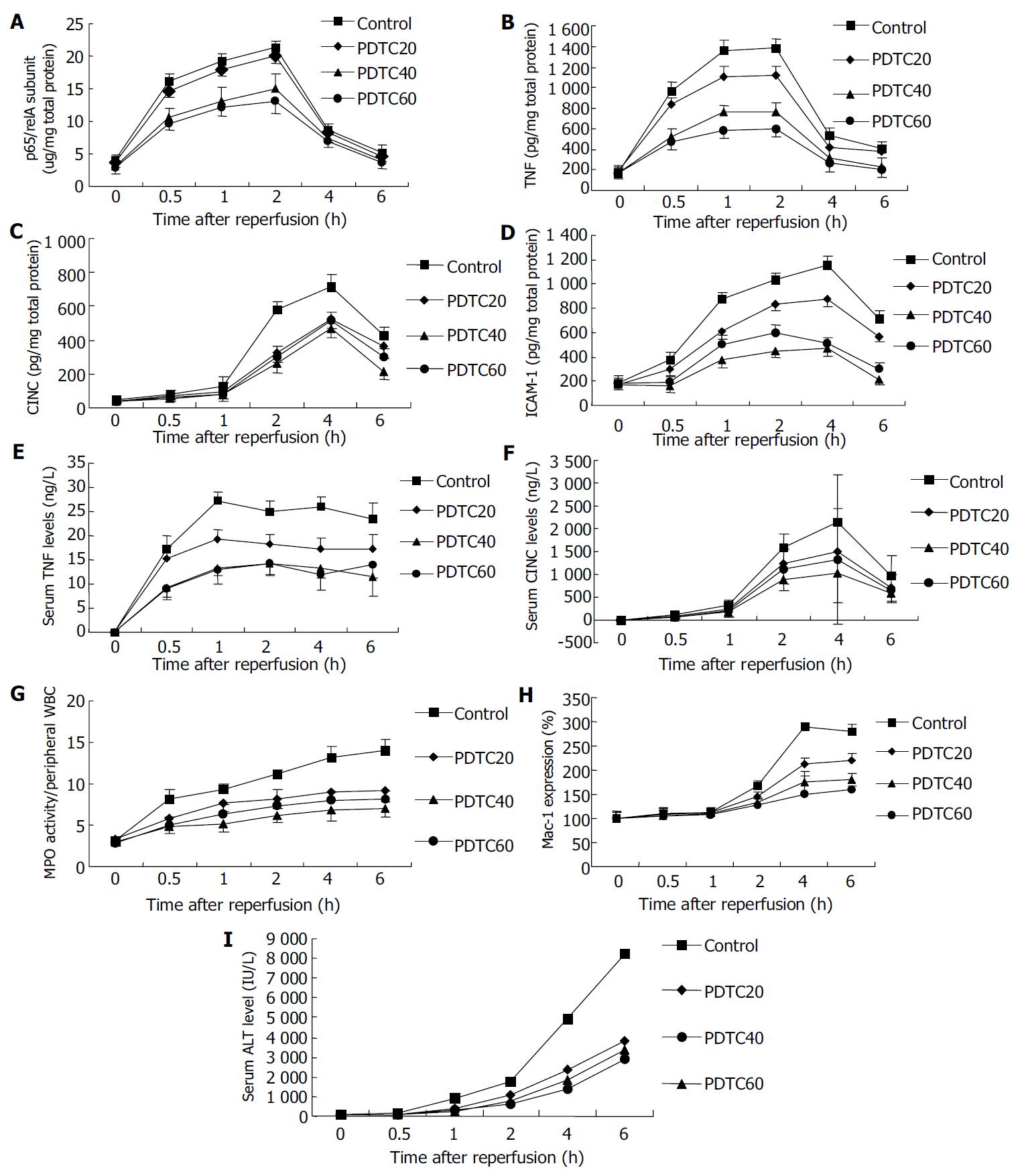
The time course of NF-κB activity in liver tissue was established by evaluating p65/relA subunits in nuclear extracts from the whole liver obtained at various time points. In the control group, the activity of NF-κB in preserved graft increased slightly 0.5 h after reperfusion, and markedly 1 h after reperfusion, peaked at 2 h of reperfusion and decreased 4 h after reperfusion (Figure 1). PDTC suppressed OLT-induced p65/relA subunit increase in a dose-dependent manner. PDTC administration did not affect the time course for maximal NF-κB activation after OLT (Figure 1).
We determined the time course of OLT-induced expression of TNF-α, CINC, and ICAM-1 in the same tissues as used for NF-κB p65 transcription factor assay. We compared TNF-α, CINC, and ICAM-1 protein levels in tissue homogenates of livers treated by PDTC with those of livers untreated by PDTC. We observed different kinetics in the expression of inflammatory mediators. TNF-α expression was maximal 1 to 2 h after reperfusion, whereas CINC and ICAM-1 expression peaked 2 to 4 h after reperfusion (Figure 1). This was preceded by NF-κB activation.
Pretreatment with 20, 40, and 60 mg/kg of PDTC suppressed the expression of these proteins, although the response was variable (Figure 1). Suppression by PDTC of OLT-induced expression of these proteins did not show a clear dose-dependency. Maximal inhibition of CINC and ICAM-1 expression was observed at a PDTC concentration of 40 mmol/L. Maximal inhibition of TNF-α expression was observed at a PDTC concentration of 60 mmol/L.
The effects of PDTC on plasma TNF-α and CINC are shown in Figure 1. Pretreatment with PDTC at concentrations of 20, 40, and 60 mmol/L reduced the OLT-induced plasma TNF-α levels to 66%, 51%, 46% and plasma CINC levels to 70%, 48%, and 61% 4 h after reperfusion, respectively.
We utilized the MPO biochemical assay to measure tissue neutrophil infiltration during the course of reperfusion. In control transplant livers, MPO/WBC was significantly elevated within 30 min of reperfusion than that in non-reperfusion liver tissue and remained significantly elevated throughout the 6 h of reperfusion. PDTC reduced the reperfusion-induced PMN accumulation (Figure 1). Maximal inhibition was observed at a PDTC concentration of 40 mmol/L that correlated with the inhibition of OLT- induced intrahepatic CINC and ICAM-1 expression.
The CD11b content in circulating neutrophils was quantitatively evaluated by flow cytometric analysis at 0, 0.5, 1, 2, 4 and 6 h after the onset of reperfusion. In the control group, Mac-1 expression at the control time point (0 h) showed low levels of staining for CD11b [mean fluorescence intensity (MFI) = 76.75 ± 19.15], which reached 167% of baseline levels at 2 h, and 289% of baseline levels at 4 h, respectively, and remained constantly elevated at 6 h (281%) after reperfusion in control group (Figure 1).
PDTC inhibited Mac-1 expression in circulating neutrophils in a dose-dependent manner (Figure 1). Maximal inhibition was observed at a PDTC concentration of 60 mmol/L that correlated with the inhibition of OLT-induced systemic TNF-α response but not systemic CINC response, indicating that TNF-α was a much more strong inflammatory mediator for neutrophil activation.
Serum ALT levels increased 0.5 h after reperfusion, remained greatly elevated throughout the 6 h reperfusion, and increased significantly in the 18 h and 24 h cold-preserved groups, compared with those in the 6 h group during the 6 h observation (Figure 1). PDTC ameliorated liver function after transplantation, the maximal increase was observed at a PDTC concentration of 40 mmol/L. PDTC administration did not affect the time course for maximal graft injury after OLT (Figure 1).
In this study, we extended our investigation on the pathogenesis of neutrophilic graft inflammation following rat orthotopic liver transplantation to assess the potential contribution of transcription factor after NF-κB[6]. Since transcription factors could determine cellular phenotypes by specifying protein production[22], it is particularly interesting to relate changes in activation of transcription factors such as NF-κB, to expression of a specific protein and evolution of biological relevant end points[23-25]. We studied the expression of TNF-α, ICAM-1 and CINC because of their critical roles in the reperfusion-induced inflammatory responses. We demonstrated that reperfusion-induced NF-κB activation in vivo preceded the expression of TNF-α, CINC and ICAM-1. In our rat model of orthotopic liver transplantation, we observed a link among NF-κB activation, systemic and intrahepatic TNF-α and CINC responses, intrahepatic ICAM-1 expression, neutrophilic inflammation, and graft injury. In addition, we found that inhibition of NF-κB activation with PDTC decrease local TNF-α, CINC and ICAM-1 expression, ameoliated neutrophilic inflammation, and improve graft function. These observations support the hypothesis that NF-κB activation is critical for early graft injury in liver transplantation.
Dithiocarbamates, such as PDTC, represent a class of antioxidants reported to be potent inhibitors of NF-κB and are capable of inhibiting the inflammatory process associated with the activation of NF-κB[26,27]. The most effective inhibitor of NF-κB appears to be the pyrrolidine derivative of dithiocarbamate (PDTC) as a result of its ability to traverse the cell membrane and its prolonged stability in solution at physiological pH. PDTC, as well as other antioxidants, may inhibit NF-κB by suppressing the production of intracellular reactive oxygen species[26]. But it is well established that inhibition of NF-κB is a major mechanism of the anti-inflammatory actions of PDTC[27]. In our study, PDTC probably blocked neutrophillic inflammation in part by diminishing NF-κB -dependent transcription of CINC and ICAM-1 expression. Blocking NF-κB activation might also diminish the transcription of a variety of other genes involved in the production of inflammatory mediators.
It was interesting that PDTC at 60 mmol/L caused less inhibition of OLT-induced expression of CINC and ICAM-1 than PDTC at 40 mmol/L PDTC, although the higher concentration exerted greater inhibition of NF-κB activation. This dissociation between inhibition of NF-κB activation and ICAM-1, CINC expression might be explained by involvement of AP-1 in mediating the response[14]. Thus, it is likely that 60 mmol/L PDTC inhibited the NF-κB-mediated ICAM-1 and CINC expression, but augmented AP-1-mediated transcription of the ICAM-1 and CINC expression, the inhibitory effect of PDTC mediated through NF-κB inhibition was consequently offset by the AP-1-mediated stimulatory effect, which needs further work to identify. In addition, PDTC at 60 mmol/L caused less neutrophil accumulation in the grafts, but the neutrophil activity correlated with less improvement of graft injury than 40 mmol/L PDTC, implying that local accumulation of activated neutrophils is the definitive factor of local neutrophilic inflammation.
The findings that PDTC administration ameoliated liver injury at 0.5 h and 1 h after reperfusion, as measured by serum ALT levels, were also noted to have some effects on neutrophil-independent phase of liver injury associated with OLT. In this study, neutrophil sequestration occurred quickly within 0.5 h after reperfusion, with no changes in Mac-1 and L-selectin expression at 0.5 h and 1 h time points. Previous studies demonstrated that increased expression of Mac-1[28] and shedding of L-selectin[29] were important to neutrophil-mediated injury. Hepatic injury at this time point was neutrophil-independent and was mediated by cytosolic xanthine oxidase and mitochondria in damaged hepatocytes[30], the most relevant oxidant stress occurred initially in the vasculature and was produced by Kupffer cells[31]. Our data suggested that there was a protective effect of PDTC in the liver at 0.5 h and 1 h after reperfusion, which might be mediated by antioxidants or Kupffer cells. In this study, the data showed that PDTC inhibited intrahepatic TNF-α expression in a dose-dependent manner. A study showed that TNF-α was probably from activated Kupffer cells in cold-preserved livers[32], suggesting that he presence of primed Kupffer cells during extended cold preservation might result in overresponse and excessive production of TNF-α in the initial phase of reperfusion. The decreased expression of TNF-α in PDTC treatment group might in part reflect the inhibition of Kupffer cells.
In summary, the pathophysiological mechanisms of the protective effect of PDTC in vivo, appear to be pluripotent, comprising both antioxidative properties and inhibition of NF-κB. Inhibiting early NF-κB activity can protect against early graft injury in prolonged cold preservation after liver transplantation.
We thank Dr. Jun-Bao Cheng, Li-Hua Zang from Drum Tower Hospital, Nanjing, for invaluable technical assistance.
| 1. | Clavien PA, Harvey PR, Strasberg SM. Preservation and reperfusion injuries in liver allografts. An overview and synthesis of current studies. Transplantation. 1992;53:957-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 617] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 2. | Furukawa H, Todo S, Imventarza O, Casavilla A, Wu YM, Scotti-Foglieni C, Broznick B, Bryant J, Day R, Starzl TE. Effect of cold ischemia time on the early outcome of human hepatic allografts preserved with UW solution. Transplantation. 1991;51:1000-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 170] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Natori S, Selzner M, Valentino KL, Fritz LC, Srinivasan A, Clavien PA, Gores GJ. Apoptosis of sinusoidal endothelial cells occurs during liver preservation injury by a caspase-dependent mechanism. Transplantation. 1999;68:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 176] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Sakamoto N, Sun Z, Brengman ML, Maemura K, Ozaki M, Bulkley GB, Klein AS. Hepatic reticuloendothelial system dysfunction after ischemia-reperfusion: role of P-selectin-mediated neutrophil accumulation. Liver Transpl. 2003;9:940-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Okaya T, Lentsch AB. Cytokine cascades and the hepatic inflammatory response to ischemia and reperfusion. J Invest Surg. 2003;16:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Gu XP, Jiang Y, Xu FT, Qiu YD, Ding YT. Effect of cold-ischemia time on nuclear factor-kappaB activation and inflammatory response in graft after orthotopic liver transplantation in rats. World J Gastroenterol. 2004;10:1000-1004. [PubMed] |
| 7. | Kato A, Yoshidome H, Edwards MJ, Lentsch AB. Regulation of liver inflammatory injury by signal transducer and activator of transcription-6. Am J Pathol. 2000;157:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Arii S, Teramoto K, Kawamura T. Current progress in the understanding of and therapeutic strategies for ischemia and reperfusion injury of the liver. J Hepatobiliary Pancreat Surg. 2003;10:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Bradham CA, Stachlewitz RF, Gao W, Qian T, Jayadev S, Jenkins G, Hannun Y, Lemasters JJ, Thurman RG, Brenner DA. Reperfusion after liver transplantation in rats differentially activates the mitogen-activated protein kinases. Hepatology. 1997;25:1128-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 160] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Xu J, Yang Z, Zeng J. Role of NF-kappa B in liver ischemia reperfusion injury of rats. J Huazhong Univ Sci Technolog Med Sci. 2003;23:158-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Takahashi Y, Ganster RW, Ishikawa T, Okuda T, Gambotto A, Shao L, Murase N, Geller DA. Protective role of NF-kappaB in liver cold ischemia/reperfusion injury: effects of IkappaB gene therapy. Transplant Proc. 2001;33:602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Tsoulfas G, Geller DA. NF-kappaB in transplantation: friend or foe? Transpl Infect Dis. 2001;3:212-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Bradham CA, Schemmer P, Stachlewitz RF, Thurman RG, Brenner DA. Activation of nuclear factor-kappaB during orthotopic liver transplantation in rats is protective and does not require Kupffer cells. Liver Transpl Surg. 1999;5:282-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Takahashi Y, Ganster RW, Gambotto A, Shao L, Kaizu T, Wu T, Yagnik GP, Nakao A, Tsoulfas G, Ishikawa T. Role of NF-kappaB on liver cold ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1175-G1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Ricciardi R, Kim RD, McDade TP, Perugini RA, Veal TM, Quarfordt SH, Callery MP, Chari RS, Meyers WC. NFkappaB expression during cold ischemia correlates with postreperfusion graft function. J Surg Res. 2000;93:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Bradham CA, Schemmer P, Stachlewitz RF, Thurman RG, Brenner DA. Activation of nuclear factor-kappaB during orthotopic liver transplantation in rats is protective and does not require Kupffer cells. Liver Transpl Surg. 1999;5:282-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Kamada N, Calne RY. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 1979;28:47-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 535] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 18. | Jiang Y, Gu XP, Qiu YD, Sun XM, Chen LL, Zhang LH, Ding YT. Ischemic preconditioning decreases C-X-C chemokine expression and neutrophil accumulation early after liver transplantation in rats. World J Gastroenterol. 2003;9:2025-2029. [PubMed] |
| 19. | Chandrasekar B, Streitman JE, Colston JT, Freeman GL. Inhibition of nuclear factor kappa B attenuates proinflammatory cytokine and inducible nitric-oxide synthase expression in postischemic myocardium. Biochim Biophys Acta. 1998;1406:91-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Schmekel B, Karlsson SE, Linden M, Sundström C, Tegner H, Venge P. Myeloperoxidase in human lung lavage. I. A marker of local neutrophil activity. Inflammation. 1990;14:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Vuorte J, Jansson SE, Repo H. Standardization of a flow cytometric assay for phagocyte respiratory burst activity. Scand J Immunol. 1996;43:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Lee JI, Burckart GJ. Nuclear factor kappa B: important transcription factor and therapeutic target. J Clin Pharmacol. 1998;38:981-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 275] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 23. | Zwacka RM, Zhang Y, Zhou W, Halldorson J, Engelhardt JF. Ischemia/reperfusion injury in the liver of BALB/c mice activates AP-1 and nuclear factor kappaB independently of IkappaB degradation. Hepatology. 1998;28:1022-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 143] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Miyoshi H, Rust C, Guicciardi ME, Gores GJ. NF-kappaB is activated in cholestasis and functions to reduce liver injury. Am J Pathol. 2001;158:967-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Kato A, Edwards MJ, Lentsch AB. Gene deletion of NF-kappa B p50 does not alter the hepatic inflammatory response to ischemia/reperfusion. J Hepatol. 2002;37:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Hong HJ, Wu CC, Yen MH. Pyrrolidine dithiocarbamate improves the septic shock syndromes in spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 1998;25:600-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Liu SF, Ye X, Malik AB. Inhibition of NF-kappaB activation by pyrrolidine dithiocarbamate prevents In vivo expression of proinflammatory genes. Circulation. 1999;100:1330-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 281] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 28. | Vollmar B, Menger MD, Glasz J, Leiderer R, Messmer K. Impact of leukocyte-endothelial cell interaction in hepatic ischemia-reperfusion injury. Am J Physiol. 1994;267:G786-G793. [PubMed] |
| 29. | Lawson JA, Burns AR, Farhood A, Lynn Bajt M, Collins RG, Smith CW, Jaeschke H. Pathophysiologic importance of E- and L-selectin for neutrophil-induced liver injury during endotoxemia in mice. Hepatology. 2000;32:990-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Hines IN, Harada H, Wolf R, Grisham MB. Superoxide and post-ischemic liver injury: potential therapeutic target for liver transplantation. Curr Med Chem. 2003;10:2661-2667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Schauer RJ, Bilzer M, Kalmuk S, Gerbes AL, Leiderer R, Schildberg FW, Messmer K. Microcirculatory failure after rat liver transplantation is related to Kupffer cell-derived oxidant stress but not involved in early graft dysfunction. Transplantation. 2001;72:1692-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Arii S, Monden K, Adachi Y, Zhang W, Higashitsuji H, Furutani M, Mise M, Fujita S, Nakamura T, Imamura M. Pathogenic role of Kupffer cell activation in the reperfusion injury of cold-preserved liver. Transplantation. 1994;58:1072-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
Edited by Kumar M and Wang XL Proofread by Xu FM