Published online Dec 15, 2004. doi: 10.3748/wjg.v10.i24.3564
Revised: April 13, 2004
Accepted: April 20, 2004
Published online: December 15, 2004
AIM: Dendritomas formed by fusing cancer cells to dendritic cells have already been applied to clinical treatment trial of several types of cancers. Dendritic cells for the fusion in most trials and experiments were from blood monocytes in standard 7-d protocol culture, which requires 5-7 d of culture with granulocyte-macrophage–colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4), followed by 2-3 d of activation with a combination of proinflammatory mediators such as tumor necrosis factor α (TNFα ), interleukin-1β (IL-1β ), interleukin-6 (IL-6) and prostaglandin E2 (PGE2). One study showed that mature monocyte-derived dendritic cells could be obtained within 48 h of in vitro culture with the same protocol as standard 7-d culture and referred to as FastDCs. Here we aimed to fuse human hepatocellular carcinoma cell line HCCLM3 cells with mature monocyte-derived dendritic cells within 48 h of in vitro culture (FastDC).
METHODS: HCCLM3 cells were cultured in RPMI 1640 with 150 mL/L fetal calf serum (FCS). CD14+ monocytes from healthy human peripheral blood were purified with MACS CD14 isolation kit and cultured in six-well plates in fresh complete DC medium containing RPMI-1640, 20 mL/L heat inactivated human AB serum, 2 mmol/L L-glutamine, 100 µg/mL gentamicin, 1000 U/mL GM-CSF and 500 U/mL IL-4 for 24 h, then proinflammatory mediators such as TNFα (1000 U/mL), IL-1β (10 ng/mL), IL-6 (10 ng/mL) and PGE2 (1 μg/mL) were supplemented for another 24 h, and thus mature FastDCs were generated. HCCLM3 cells and FastDCs were labeled with red fluorescent dye PKH26-GL and green fluorescent dye PKH67-GL respectively. After the red fluorescent-stained HCCLM3 cells were irradiated with 50 Gy, FastDCs and irradiated HCCLM3 cells were fused in 500 mL/L polyethylene glycol(PEG) + 100 mL/L dimethyl sulfoxide (DMSO) to generate novel dendritomas. The FastDCs and novel dendritomas were immunostained with anti-CD80, anti-CD86, anti-CD83, anti-HLA-DR mAbs and analyzed by fluorescence-activated cell sorting (FACS). Novel dendritomas were nucleus-stained with Hoechst 33258 and analyzed by confocal laser scanning microscopy.
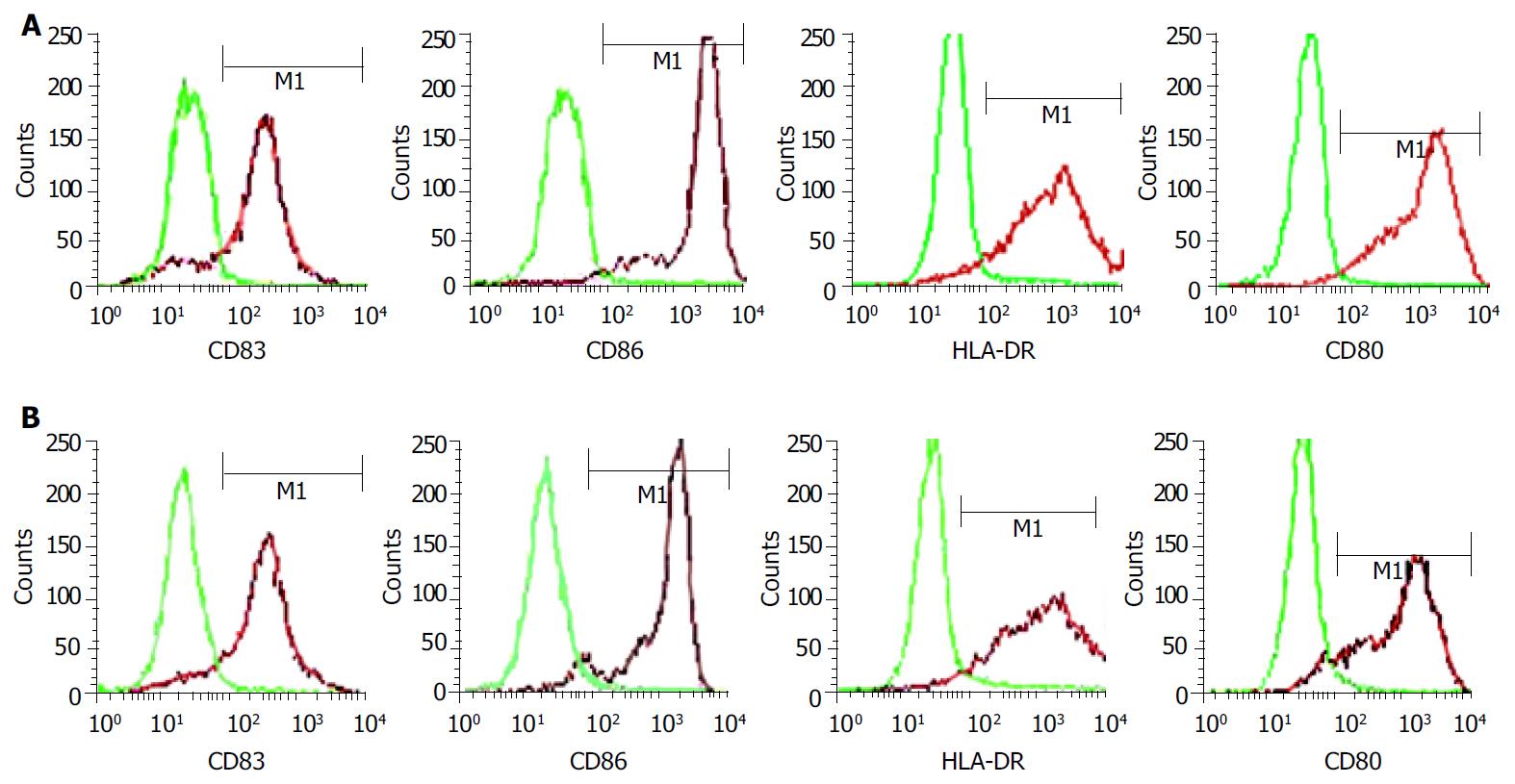
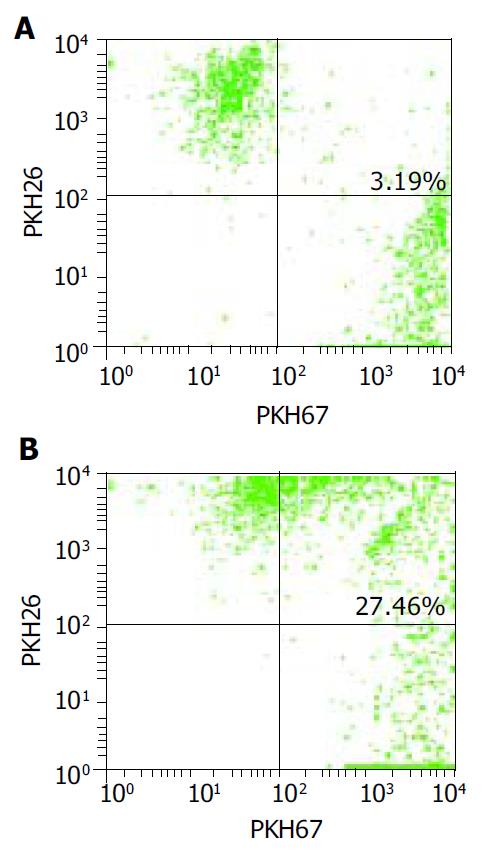
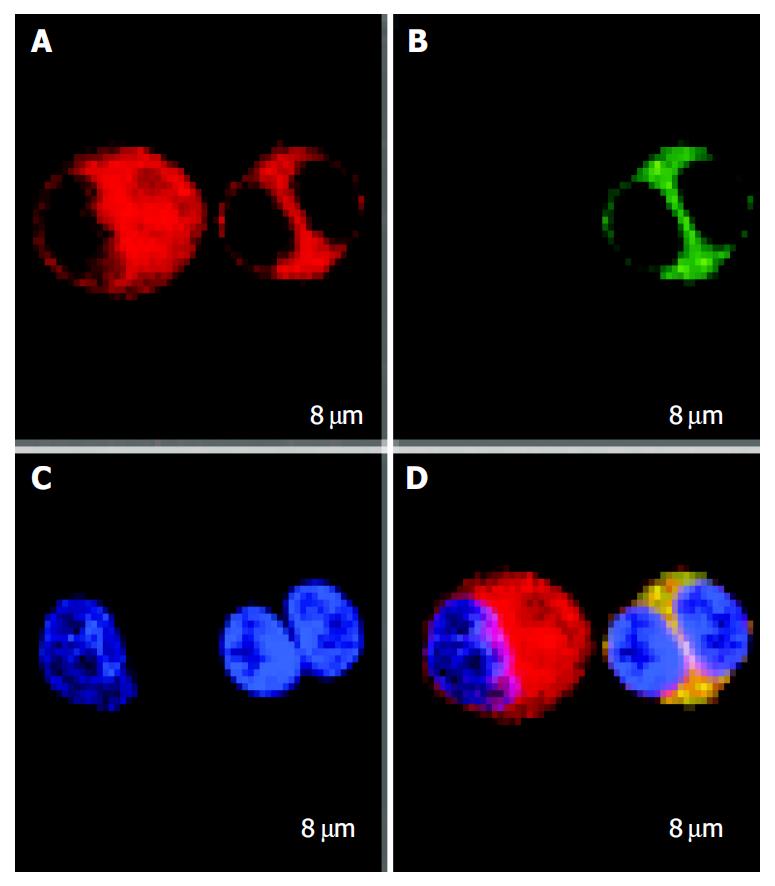
RESULTS: Mature FastDCs with highly expressed surface markers CD80, CD86, CD83 and HLA-DR were generated within 48 h in vitro. Novel dendritomas with dual red-green fluorescence were constructed fast and successfully, and FACS analysis showed that the fusion efficiency was 24.27% and the novel dendritomas expressed the same activation markers as FastDCs. Confocal laser scanning microscopy analysis showed representative images of dendritomas.
CONCLUSION: Dendritomas can be formed fast with mature FastDCs from healthy human peripheral blood monocytes (PBMC) by incubation with GM-CSF and IL-4 for 24 h and by activation with proinflammatory mediators for an additional period of 24 h. Owing to shorter time required for in vitro DCs development, the generation of these novel dendritomas reduced labor and cost. This rapid method for formation of dendritomas may represent a new strategy for immunotherapy of hepatocellular carcinoma.
-
Citation: Guan X, Peng JR, Yuan L, Wang H, Wei YH, Leng XS. A novel, rapid strategy to form dendritomas from human dendritic cells and hepatocellular carcinoma cell line HCCLM3 cells using mature dendritic cells derived from human peripheral blood CD14+ monocytes within 48 hours of
in vitro culture. World J Gastroenterol 2004; 10(24): 3564-3568 - URL: https://www.wjgnet.com/1007-9327/full/v10/i24/3564.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i24.3564
Dendritic cells (DCs) are professional antigen-presenting cells (APCs) and play key roles in initiating and managing specific primary immune responses, including the activation of tumor-reactive cytotoxic T cells (CTLs)[1,2]. The fusion of DCs with tumor cells among various tumor vaccination strategies focusing on DCs has become a promising approach[3,4]. At present, most experimental and clinical studies rely on the in vitro development of DCs from CD34+ progenitor cells or blood monocytes[5-7]. Commonly, according to standard 7-d protocol, blood monocytes were cultured for 5-7 d with GM-CSF and IL-4 to develop immature DCs which were activated for another 2-3 d with monocyte-conditioned media (MCM) or a combination of proinflammatory mediators such as TNFα , IL-1β , IL-6 and PGE2 so that mature DCs with full T stimulatory capacity were obtained[8]. It was reported that mature DCs in 48 h in vitro culture with the same combination of proinflammatory mediators as standard 7-d protocol’s were obtained and referred to as FastDCs[8]. The report indicated that FastDCs were as effective as monocyte-derived DCs from standard 7-d protocol culture in stimulating primary antigen-specific Th1-type immune responses[8]. Here we aimed to fuse human hepatocellular carcinoma cell line HCCLM3 cells with mature dendritic cells (FastDCs) within 48 h of in vitro culture.
All reagents were obtained from the indicated sources. GM-CSF, TNFα , IL-1β , IL-6 were purchased from R&D Systems (Minneapolis, USA), IL-4 from Promega (USA), PKH26-GL, PKH67-GL, 500 mL/L PEG + 100 mL/L DMSO, gentamicin, Hoechst 33258 and PGE2 from Sigma-Aldrich China Inc. (Shanghai, China), Fetal calf serum (FCS), RPMI-1640 medium, L-glutamine, penicillin and streptomycin from Invitrogen (USA). All mAbs (anti-HLA-DR, APC-conjugated; anti-CD80, Cy-Chrome-conjugated; anti-CD86, APC-conjugated; anti-CD83, APC-conjugated) were obtained from BD PharMingen (USA), and CD14 isolation kit from Miltenyi Biotec. (Bergisch-Gladbach, Germany), and Ficoll-Hypaque from Pharmacia (Sweden), Human AB serum from Chuanye Inc.(Tianjin, China). Human HCCLM3 cell line was a gift from Professor Zhao-You Tang in Liver Cancer Institute (Zhongshan Hospital, Fudan University).
Culture of hepatocellular carcinoma HCCLM3 cells Human HCCLM3 cells were grown in complete culture medium containing RPMI-1640, 150 mL/L heat-inactivated FCS, 2 mmol/L L-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin.
Isolation and culture of CD14+ monocytes Peripheral blood mononuclear cells (PBMC) were isolated from peripheral blood of healthy donors by Ficoll-Hypaque (1.077 g) density gradient centrifugation. CD14+ cells of the PBMC were separated by performing positive selection with CD14+ micro magnetic beads according to the manufacturer’s instructions and subsequently cultured in six-well plates (1 × 106 cells/mL) in fresh complete DC medium ( RPMI-1640, 20 mL/L heat inactivated human AB serum, 2 mmol/L L-glutamine, 100 μg/mL gentamicin, supplemented with 1000 U/mL GM-CSF and 500 U/mL IL-4) for 24 h, followed by incubation with a combination of proinflammatory mediators such as TNF-α (1000 U/mL), IL-1β (10 ng/mL), IL-6 (10 ng/mL) and PGE2 (1 μg/mL) for another 24 h to produce FastDCs.
Analysis of FastDCs by fluorescence-activated cell sorting FastDCs were immunostained with the following mAbs: anti-CD80, anti-CD86, anti-CD83, anti-HLA-DR. Surface marker analysis was performed by fluorescence-activated cell sorting (FACS).
Fluorescent labeling of dendritic cells and human HCCLM3 cells Commercial fluorescent cell linker kits PKH67-GL and PKH26-GL were used for membrane labeling of FastDCs and HCCLM3 cells. The FastDCs were labeled fluorescent green with PKH67-GL and HCCLM3 cells were labeled fluorescent red with PKH26-GL. The whole procedure was performed at 25 °C. The cells to be stained were washed with serum-free RPMI-1640. The cell suspension was centrifuged at 400 r/min for 5 min to produce a cell pellet. The supernatant was removed, leaving less than 25 μL medium on the pellet. One milliliter of diluent C was added to resuspend the cells. Then 4 × 10-6 molar dyes (× 2) were prepared with diluent C immediately before staining. The cells in diluent C were added rapidly to one milliliter of 2 × dye. The cells and dye were mixed by gentle pipetting. The mixture was incubated at 25 °C for 5 min. The staining process was stopped by adding an equal volume of FCS and incubating for 1 min. The stained cells were diluted with an equal volume of complete culture medium and centrifuged at 400 r/min for 10 min and removed for at least three washes. Then the cells were resuspended in fresh complete medium. The staining efficiency was monitored by fluorescent microscopy.
Fusion of dendritic cells and human HCCLM3 cells After the red fluorescent-stained HCCLM3 cells were irradiated with 50 Gy, the DCs and irradiated HCCLM3 cells were fused together by mixing two cell types at an 1:1 ratio in a 50 mL conical centrifugation tube. One milliliter of fusogen containing 500 mL/L PEG + 100 mL/L DMSO was added to the mixture by dropping for 1-1.5 min. Nine milliliters of serum-free RPMI-1640 with 25 mmol/L Hepes was added to the mixture for over 5 min. Forty milliliters of serum-free RPMI-1640 was added to the cell mixture and the mixture was pelleted by centrifugation at 800 r/min for 10 min. After the supernatant was removed, the cells were resuspended in one milliliter of complete culture medium.
Sort and analysis of novel dendritomas The fused cells were resuspended in phosphate-buffered saline for detection of dual fluorescent dendritomas by FACS.
The novel dendritomas were sorted based on dual green and red fluorescence using a FACS caliber cell sorter (Becton Dickinson, USA). The cells were centrifuged at 800 r/min for 15 min. After the supernatant was removed, the cells were immunostained with the same mAbs as for FastDCs and activation markers were analyzed.
Analysis of novel dendritomas by confocal laser scanning microscopy The fused cells were resuspended in phosphate-buffered saline and centrifuged at 400 r/min for 10 min. After the supernatant was removed, Hoechst 33258 for nucleus staining was dropped in and the cells were resuspended in phosphate-buffered saline 5 min later and centrifuged at 400 r/min for 10 min again. After the cells were fixed with 10 g/L paraformaldehyde, nucleus-stained cells were resuspended in phosphate-buffered saline for analysis of dendritomas by confocal laser scanning microscopy.
Monocytes were enriched from PBMC by CD14-positive selection with MACS and subsequently cultured with GM-CSF and IL-4 for 48 h. Proinflammatory mediators such as TNFα , IL-1β , IL-6 and PGE2 were added to accelerate DCs maturation after 24 h of culture with GM-CSF and IL-4. The cells displayed mature DC activation markers such as CD83++, CD80++, CD86++and HLA-DR++within 48 h (Figure 1A) and formed long cytoplasmic protrusions typical of mature DCs, while monocytes cultured with GM-CSF and IL-4 alone for 48 h displayed and maintained monocyte-like morphology. Using this two-step differentiation strategy, a large number of mature and viable DCs ( about 30% of the initial population of monocytes) could be obtained.
After FastDCs and HCCLM3 cells were labeled with PKH67-GL and PKH26-GL respectively, the cells were examined under fluorescent microscopy and more than 95% of the cells were stained successfully.
FastDCs were stained fluorescent green with PKH67-GL and HCCLM3 cells were stained fluorescent red with PKH26-GL. The stained two cell types were fused together by admixing them and dropping fusogen. The novel dendritomas would take on dual fluorescence. FACS analysis showed that the percentage of red and green dual fluorescent dendritomas in the fused cell mixture was 24.27%, which represented fusion efficiency (Figure 2) and the novel dendritomas expressed the same markers as FastDCs (Figure 1B).
FastDCs and HCCLM3 cells were stained with PKH67-GL and PKH26-GL respectively. After FastDCs and HCCLM3 cells were fused, nuclear counterstaining was performed using Hoechst 33258. Under confocal laser scanning microscopy, FastDCs were detected as green cells and HCCLM3 cells were detected as red and the representative image of a dendritoma was a dual fluorescent cell with two blue nuclei (Figure 3).
As highly specialized antigen-presenting cells, DCs constitute a unique system of cells that induce and control immune responses. Owing to their unique ability to capture and present antigens, thereby inducing and managing immune responses, DCs have become attractive vectors and targets for immunological intervention in numerous diseases and represent optimal candidates, especially for cancer immunotherapy. So far, various tumor vaccination strategies have been developed based on the loading of DCs with tumor-associated antigens (TAAs)[9,10], including defined peptides of known sequences[11-13], undefined acid-eluted peptides from autologous tumors[14], whole tumor lysates[15], tumor cell-derived RNA et al[16]. Another promising alternative is the fusion of DCs with tumor cells[3,4]. This approach is based on the idea that multiple TAAs are endogenously processed and presented by MHC class I molecules, thereby stimulating tumor-specific CTLs[17] .
In 1997, Gong et al[3] reported that they fused breast carcinoma cells with DCs to produce dendritomas which were capable of presenting antigens effectively and inducing antitumor-specific CTLs. Afterwards, a number of experimental and clinical trials with significant effects on several types of cancers such as melanoma, leukemia, glioma, gastric carcinoma, myeloma, renal cell carcinoma and ovarian carcinoma were reported[18-31].
At present, DCs, for experimental and clinical fusion trial, were obtained mainly from in vitro standard 7-d protocol culture. In fact, the kinetics of DCs differentiation from blood monocytes under physiologic conditions might not be reflected by current standard protocols for the in vitro development of DCs[8]. One research showed that a subpopulation of blood monocytes differentiated into DCs within 48 h in a model simulating transendothelial migration into lymphatic vessels[31]. Dauer et al[8]reported that they cultured blood monocytes within 48 h in vitro with the same combination of proinflammatory mediators as standard 7-d protocol and obtained mature DCs with their ability similar to standard protocol DCs in stimulating primary antigen-specific Th1-type immune responses, and inducing the production of IFNγ and activating autologous naïve T cells, and the DCs were referred to as FastDCs. We cultured and obtained FastDCs with activation markers using the same methods and successfully constructed dendritomas by fusing FastDCs with HCCLM3 cells. Our study showed that the fusion of the two cells was feasible and the novel dendritomas expressed the same surface markers as FastDCs. Compared with common standard protocol methods, this new strategy not only simplified the process and reduced labor, cost and time for the whole experiment procedure, but also may be less disrupted by microbial contamination.
In conclusion, our novel strategy may facilitate the use of dendritomas in clinical trials of cancer immunotherapy
We express our gratitude to Professor Zhao-You Tang in Liver Cancer Institute of Zhongshan Hospital, Fudan University for his gift.
| 1. | Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10867] [Cited by in RCA: 10811] [Article Influence: 386.1] [Reference Citation Analysis (0)] |
| 2. | Kurokawa T, Oelke M, Mackensen A. Induction and clonal expansion of tumor-specific cytotoxic T lymphocytes from re-nal cell carcinoma patients after stimulation with autologous dendritic cells loaded with tumor cells. Int J Cancer. 2001;91:749-756. [DOI] [Full Text] |
| 3. | Gong J, Chen D, Kashiwaba M, Kufe D. Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells. Nat Med. 1997;3:558-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 434] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 4. | Soruri A, Fayyazi A, Neumann C, Schlott T, Jung T, Matthes C, Zwirner J, Riggert J, Peters JH. Ex vivo generation of human anti-melanoma autologous cytolytic T cells by dentritic cell/melanoma cell hybridomas. Cancer Immunol Immunother. 2001;50:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1165] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 6. | Romani N, Gruner S, Brang D, Kämpgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch PO, Steinman RM, Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1414] [Cited by in RCA: 1383] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 7. | Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109-1118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3910] [Cited by in RCA: 4000] [Article Influence: 125.0] [Reference Citation Analysis (0)] |
| 8. | Dauer M, Obermaier B, Herten J, Haerle C, Pohl K, Rothenfusser S, Schnurr M, Endres S, Eigler A. Mature dendritic cells derived from human monocytes within 48 hours: a novel strategy for dendritic cell differentiation from blood precursors. J Immunol. 2003;170:4069-4076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 247] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 9. | Thurner B, Haendle I, Röder C, Dieckmann D, Keikavoussi P, Jonuleit H, Bender A, Maczek C, Schreiner D, von den Driesch P. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;190:1669-1678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 928] [Cited by in RCA: 881] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 10. | Mackensen A, Herbst B, Chen JL, Kohler G, Noppen C, Herr W, Spagnoli GC, Cerundolo V, Lindemann A. Phase I study in melanoma patients of a vaccine with peptide-pulsed dendritic cells generated in vitro from CCD34 hematopoietic progenitor cells. Int J Cancer. 2000;86:385-392. [DOI] [Full Text] |
| 11. | Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Celluzzi C, Falo LD, Melief CJ, Ildstad ST, Kast WM, Deleo AB. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med. 1995;1:1297-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 820] [Cited by in RCA: 786] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 12. | Porgador A, Gilboa E. Bone marrow-generated dendritic cells pulsed with a class I-restricted peptide are potent inducers of cytotoxic T lymphocytes. J Exp Med. 1995;182:255-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 277] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 13. | Celluzzi CM, Mayordomo JI, Storkus WJ, Lotze MT, Falo LD. Peptide-pulsed dendritic cells induce antigen-specific CTL-mediated protective tumor immunity. J Exp Med. 1996;183:283-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 547] [Cited by in RCA: 538] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 14. | Zitvogel L, Mayordomo JI, Tjandrawan T, DeLeo AB, Clarke MR, Lotze MT, Storkus WJ. Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines. J Exp Med. 1996;183:87-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 623] [Cited by in RCA: 612] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 15. | Fields RC, Shimizu K, Mulé JJ. Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proc Natl Acad Sci USA. 1998;95:9482-9487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 353] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 16. | Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996;184:465-472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 671] [Cited by in RCA: 680] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 17. | Van Der Bruggen P, Zhang Y, Chaux P, Stroobant V, Panichelli C, Schultz ES, Chapiro J, Van Den Eynde BJ, Brasseur F, Boon T. Tumor-specific shared antigenic peptides recognized by human T cells. Immunol Rev. 2002;188:51-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 282] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 18. | Trefzer U, Weingart G, Chen Y, Herberth G, Adrian K, Winter H, Audring H, Guo Y, Sterry W, Walden P. Hybrid cell vaccination for cancer immune therapy: first clinical trial with metastatic melanoma. Int J Cancer. 2000;85:618-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Kugler A, Stuhler G, Walden P, Zöller G, Zobywalski A, Brossart P, Trefzer U, Ullrich S, Müller CA, Becker V. Regression of human metastatic renal cell carcinoma after vaccination with tumor cell-dendritic cell hybrids. Nat Med. 2000;6:332-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 421] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 20. | Gong J, Nikrui N, Chen D, Koido S, Wu Z, Tanaka Y, Cannistra S, Avigan D, Kufe D. Fusions of human ovarian carcinoma cells with autologous or allogeneic dendritic cells induce antitumor immunity. J Immunol. 2000;165:1705-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 147] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Galea-Lauri J, Darling D, Mufti G, Harrison P, Farzaneh F. Eliciting cytotoxic T lymphocytes against acute myeloid leukemia-derived antigens: evaluation of dendritic cell-leukemia cell hybrids and other antigen-loading strategies for dendritic cell-based vaccination. Cancer Immunol Immunother. 2002;51:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Krause SW, Neumann C, Soruri A, Mayer S, Peters JH, Andreesen R. The treatment of patients with disseminated malignant melanoma by vaccination with autologous cell hybrids of tumor cells and dendritic cells. J Immunother. 2002;25:421-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Kikuchi T, Akasaki Y, Irie M, Homma S, Abe T, Ohno T. Results of a phase I clinical trial of vaccination of glioma patients with fusions of dendritic and glioma cells. Cancer Immunol Immunother. 2001;50:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 204] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 24. | Sloan AE, Parajuli P. Human autologous dendritic cell-glioma fusions: feasibility and capacity to stimulate T cells with proliferative and cytolytic activity. J Neurooncol. 2003;64:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Gong J, Koido S, Chen D, Tanaka Y, Huang L, Avigan D, Anderson K, Ohno T, Kufe D. Immunization against murine multiple myeloma with fusions of dendritic and plasmacytoma cells is potentiated by interleukin 12. Blood. 2002;99:2512-2517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Homma S, Matai K, Irie M, Ohno T, Kufe D, Toda G. Immunotherapy using fusions of autologous dendritic cells and tumor cells showed effective clinical response in a patient with advanced gastric carcinoma. J Gastroenterol. 2003;38:989-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Homma S, Toda G, Gong J, Kufe D, Ohno T. Preventive antitumor activity against hepatocellular carcinoma (HCC) induced by immunization with fusions of dendritic cells and HCC cells in mice. J Gastroenterol. 2001;36:764-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Chen D, Xia J, Tanaka Y, Chen H, Koido S, Wernet O, Mukherjee P, Gendler SJ, Kufe D, Gong J. Immunotherapy of spontaneous mammary carcinoma with fusions of dendritic cells and mucin 1-positive carcinoma cells. Immunology. 2003;109:300-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Zhang J, Zhang JK, Zhuo SH, Chen HB. Effect of a cancer vaccine prepared by fusions of hepatocarcinoma cells with dendritic cells. World J Gastroenterol. 2001;7:690-694. [PubMed] |
| 30. | Akasaki Y, Kikuchi T, Homma S, Abe T, Kufe D, Ohno T. Antitumor effect of immunizations with fusions of dendritic and glioma cells in a mouse brain tumor model. J Immunother. 2001;24:106-113. [RCA] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Randolph GJ, Beaulieu S, Lebecque S, Steinman RM, Muller WA. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998;282:480-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 614] [Article Influence: 21.9] [Reference Citation Analysis (3)] |
Co-first-authors: Xin Guan and Ji-Run Peng
Co-correspondents: Xin Guan
Edited by Kumar M and Wang XL Proofread by Xu FM