Published online Dec 1, 2004. doi: 10.3748/wjg.v10.i23.3399
Revised: April 2, 2004
Accepted: April 5, 2004
Published online: December 1, 2004
AIM: To investigate the value of transabdominal ultrasonography (US) in the preoperative staging of gastric cancer.
METHODS: A total of 198 patients with gastric cancer underwent preoperatively transabdominal US, depth of tumor infiltration was assessed in 125 patients, and lymph node metastasis was assessed in 106 patients.
RESULTS: The staging accuracy of transabdominal US was 55.6%, 75.0%, 87.3% and 71.1% in T1, T2, T3 and T4 carcinomas, respectively. The overall accuracy was 77.6%. The detection rate for pancreatic invasion and liver invasion was 77.4%, 71.4%, respectively. The sensitivity, specificity, accuracy of transabdominal US in assessment of lymph node metastasis were 77.6%, 64.1%, 72.6%, respectively. Various shapes such as round, ovoid, spindle were encountered in benign and malignant lymph nodes. Majority of both benign and malignant lymph nodes were hyperechoic and had a distinct border. Benign lymph nodes were smaller than malignant lymph nodes in length and width (P = 0.000, 0.005). Irregular shape, fusional shape, infiltrative signs, inhomogenous echo were seen mainly in malignant lymph nodes (P = 0.045, 0.006, 0.027, 0.006).
CONCLUSION: Transabdominal US is useful for preoperative staging in gastric cancer, although it is difficult to differentiate benign from malignant lymph nodes.
- Citation: Liao SR, Dai Y, Huo L, Yan K, Zhang L, Zhang H, Gao W, Chen MH. Transabdominal ultrasonography in preoperative staging of gastric cancer. World J Gastroenterol 2004; 10(23): 3399-3404
- URL: https://www.wjgnet.com/1007-9327/full/v10/i23/3399.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i23.3399
Gastric cancer is one of the most prevalent malignant tumors[1,2]. An accurate preoperative staging is helpful for the prediction of prognosis and establishment of the individualized therapy. Although endoscopy and upper gastrointestinal series with double-contrast study have significantly improved the diagnostic accuracy in gastric cancer, they neither allow the assessment of the depth of tumor infiltration nor visualize perigastric lymph nodes[3,4]. Currently, endoscopic ultrasonography (EUS) has been considered as a useful modality for the preoperative staging of gastric cancer, and the accuracy of EUS in the assessment of the depth of tumor infiltration and lymph node metastasis is 67%-92% and 63%-78%[5-11], respectively. However, EUS procedure is relatively complex, and can not be performed successfully in some patients, due to marked obstruction of gastric lumen caused by tumor or noticeable discomfort during examination.
Transabdominal ultrasonography (US) is applied widely in clinical practice. When condition of patients is suitable, transabdominal US can detect lymph nodes with a diameter of 5 mm, and the normal wall of fluid-filled stomach after the patients drank water can be described as a 5-layer structure[12-14], which contributes to the assessment of the depth of tumor infiltration. Literature on the preoperative staging with transabdominal US is sparse[15,16]. The aim of our study was to further assess the accuracy and limitations of transabdominal US in preoperative staging of the depth of tumor infiltration and lymph node metastasis.
Between January 2000 to July 2003, 198 patients with gastric cancer confirmed by endoscopic biopsy underwent transabdominal US preoperatively. Patients with preoperative transabdominal US findings and detailed operative and pathological findings were included in this study. The depth of tumor infiltration was assessed in 125 patients (78 males, 47 females, ranged 27-78 years). Lymph node metastasis was assessed in 106 patients (66 males, 40 females, ranged 31-78 years).
All patients were required to drink water to fill stomach for the assessment of the depth of tumor infiltration. Transabdominal US was performed 3-5 min after 500-700 mL boiled water was drunk. Patients were examined usually in the supine position. Sitting position, left or right lateral decubitus position might be taken for visualization of lesions according to different locations of tumors.
The wall of fluid-filled stomach is described as a 5-layer structure[12-14]. The innermost hyperechoic layer (the first layer) corresponds to the superficial mucosa, the second hypoechoic layer corresponds to the deep mucosa, the third hyperechoic layer corresponds to the submucosa plus the acoustic interface between the submucosa and muscularis propria, the fourth hypoechoic layer corresponds to the muscularis propria minus the acoustic interface between the submucosa and muscularis propria, the fifth hyperechoic layer corresponds to the subserosal fat and serosa.
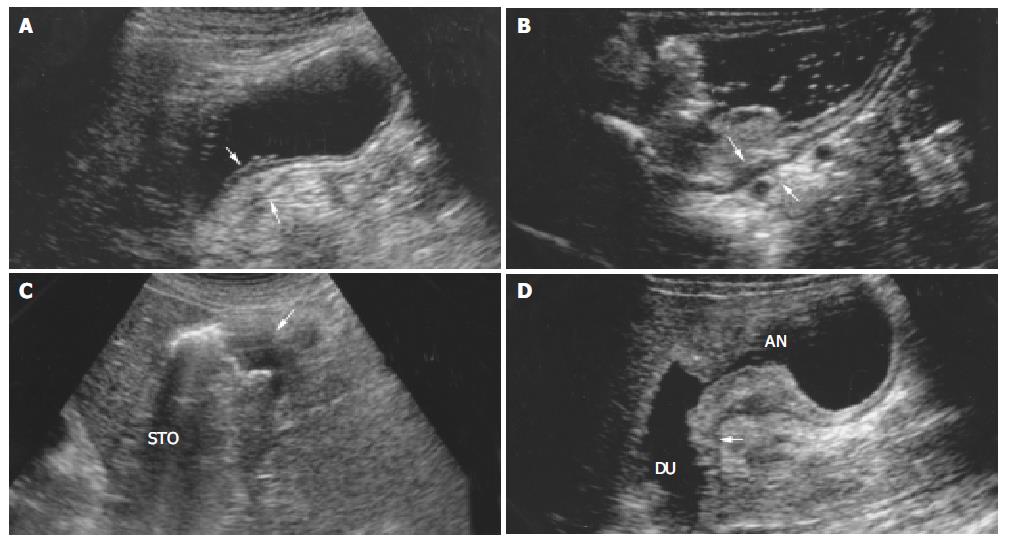
According to the TNM (UICC) classification[17], the depth of tumor infiltration was divided into 4 categories. T1 = tumor confined to mucosa or submucosa. For mucosal carcinoma, tumor located in the first and second layers, the third layers (submucosa) was intact; for submucosal carcinoma, layers 1 to 3 were interrupted or thickened and the fourth and fifth layers were normal sonographically (Figure 1A). T2 = tumor invading muscularis propria (the fourth layer) which became thicken, and the fifth layer was intact sonographically (Figure 1B). T3 = tumor invading serosa (the fifth layer), with interruption or disappearance of all layers of wall sonographically (Figure 1C). T4 = tumor invading adjacent organs which had an indistinct border and was indistinguishable from involved organs sonographically (Figure 1D).
Assessment of regional lymph node metastasis was performed in a fasting state before water was drunk for evaluation of tumor infiltration. Regional lymph nodes around the following organs or structures were examined: stomach, liver, pancreas, gallbladder, aorta, hilum of spleen, comma hepatic aorta, superior mesenteric artery, celiac artery, and recorded number, size, shape, border, and echogenicity of lymph nodes.
Lymph nodes with a length of 5 mm or greater were considered metastatic. Regional lymph node staging was classified as N0 (no lymph node metastasis) and N + (lymph node metastasis).
Lymph nodes detected with transabdominal US were divided into 3 groups according to their shapes: regular, irregular, fusional. Regular lymph nodes were classified into 3 categories of shape: spindle: width ≤ half the length of the lymph node; ovoid: width ≤ three quarters of the length of the lymph node; round: width > three quarters of the length of the lymph node[18].
The followings were considered as “infiltrative signs” of lymph nodes: ill-defined border, indistinguishable from the adjacent structures or loss of movement.
Sonographic examinations were performed with commercially available real time image units (Toshiba 6000, Aloka 2000, DU-6), and transducer frequency varied between 3.5-6.0 MHz.
Data were analyzed with SPSS10.0 software. P < 0.05 was considered statistically significant.
Table 1 summarizes the findings of transabdominal US and operative or pathological findings in 125 patients with gastric cancer. The accuracy of transabdominal US in staging of T1 carcinomas was 55.6%, 75.0% in T2 carcinomas, 87.3% in T3 carcinomas, and 71.1% in T4 carcinomas. The overall accuracy was 77.6%.
| Stage | n | Staged by operative and pathological findings | |||
| T1 | T2 | T3 | T4 | ||
| T1 | 7 | 5 | 0 | 0 | 0 |
| T2 | 34 | 3 | 12 | 7 | 2 |
| T3 | 80 | 1 | 4 | 48 | 11 |
| T4 | 38 | 0 | 0 | 0 | 32 |
| Accuracy (%) | 55.6 (5/9) | 75.0 (12/16) | 87.3 (48/55) | 71.1 (32/45) | |
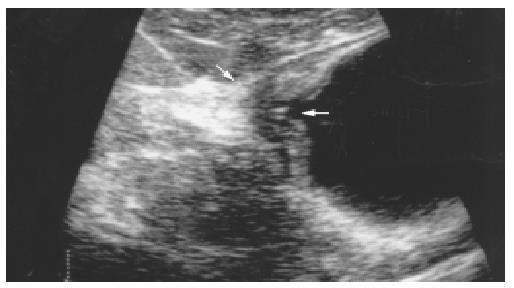
Tumors were overstaged in 8 patients. T1 carcinoma was overstaged as a T2 carcinoma in 3 patients and as a T3 carcinoma in 1 patient (Figure 2), and T2 carcinoma was overstaged as a T3 carcinoma in 4 patients. Tumors were understaged in 20 patients. T3 carcinoma was understaged as a T2 carcinoma in 7 patients. T4 carcinoma was understaged as a T2 carcinoma in 2 patients and as a T3 carcinoma in 11 patients.
Invasion of adjacent organs confirmed by operative or pathologic findings was as follows (Table 2): pancreas (31 patients), liver (7 patients), spleen and hilum of spleen (3 patients), transverse colon (3 patients), diaphragm (2 patients), and duodenum (9 patients). The detection rate for pancreas and liver invasion with transabdominal US was 77.4% and 71.4%, respectively, whereas the detection rate for the invasion of other organs was low.
| Involved organs | n | Cases detected with transabdominal US | Detection rate (%) |
| Pancreas | 31 | 24 | 77.4 |
| Liver | 7 | 5 | 71.4 |
| Spleen and hilum of spleen | 3 | 1 | 33.3 |
| Transverse colon | 3 | 1 | 33.3 |
| Diaphragm | 2 | 0 | 0 |
| Duodenum | 9 | 4 | 44.4 |
Accuracy of assessment of regional lymph metastasis Swollen lymph nodes were found pathologically in 106 patients. Lymph node metastasis was confirmed in 67 of 106 patients. Swollen lymph nodes were detected in 66 of 106 patients with transabdominal US. All lymph nodes detected sonographically had a length of 5 mm or greater, and were considered as metastatic. Of the 66 patients, lymph node metastasis was confirmed by pathological examination in 52 patients, and swollen lymph nodes were benign in the remaining 14 patients. No swollen lymph nodes were detected sonographically in 40 of 106 patients, and lymph node metastasis was confirmed in 15 of these 40 patients. The sensitivity of transabdominal US in the assessment of lymph node metastasis was 77.6% (52 of 67 patients). The specificity was 64.1% (25 of 39 patients). The accuracy was 72.6% (77 of 106 patients) (Table 3).
| Diagnosis with transabdominal US | Pathological findings (n) | |
| N+ | N0 | |
| N+ | 52 | 14 |
| N0 | 15 | 25 |
Sonographic features of benign and malignant lymph nodes
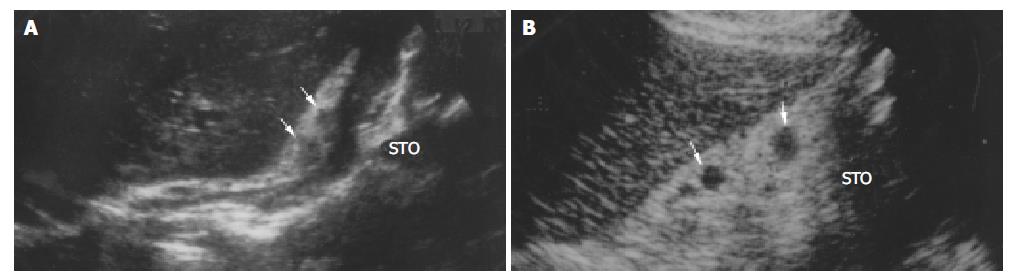
All lymph nodes in 14 of 66 patients with swollen lymph nodes detected with transabdominal US were confirmed benign (lymph node without metastasis), and transabdominal US detected 46 lymph nodes in these patients. All lymph nodes in 11 of 52 patients with lymph node metastasis were confirmed malignant (metastatic), and transabdominal US detected 48 lymph nodes in these patients. Forty-six benign lymph nodes had a mean length of 1.26 cm and a mean width of 0.84 cm, 48 malignant lymph nodes had a mean length of 1.79 cm and a mean width of 1.25 cm. According to size, shape, echogenicity, and border, sonographic features of these benign and malignant lymph nodes were further analyzed (Table 4). The length and width of benign lymph nodes were significantly smaller than those of malignant lymph nodes (P = 0.000, 0.005). Sonographic features such as irregular or fusional shape, infiltrative signs, and inhomogenous echoes were found mainly in malignant lymph nodes (Figure 3). There was a significant difference between benign and malignant lymph nodes (P = 0.045, 0.006, 0.027, 0.006).
| Sonographic feature | Pathologic findings | P | |
| Benign lymph nodes (%) | Malignant lymph nodes (%) | ||
| Length (cm) | 0.000 | ||
| < 0.5 | 0 | 0 | |
| 0.5-1.0 | 26 (56.5) | 9(18.8) | |
| 1.1-1.5 | 9 (19.6) | 16 (33.3) | |
| 1.6-2.0 | 9 (19.6) | 8 (16.7) | |
| ≥ 2.1 | 2 (4.3) | 15 (31.2) | |
| Length (cm) | 0.000 | ||
| < 0.5 | 0 | 0 | |
| 0.5-1.0 | 26 (56.5) | 9 (18.8) | |
| 1.1-1.5 | 9 (19.6) | 16 (33.3) | |
| 1.6-2.0 | 9 (19.6) | 8 (16.7) | |
| ≥ 2.1 | 2 (4.3) | 15 (31.2) | |
| Width (cm) | 0.005 | ||
| <0.5 | 5 (10.9) | 2 (4.2) | |
| 0.5-1.0 | 34 (73.9) | 22 (45.8) | |
| 1.1-1.5 | 4 (8.7) | 10 (20.8) | |
| 1.6-2.0 | 3 (6.5) | 11 (22.9) | |
| ≥ 2.1 | 0 (0) | 3 (6.3) | |
| Shape | |||
| Spindle | 9 (19.6) | 5 (10.4) | |
| Ovoid | 15 (32.6) | 10 (20.8) | |
| Round | 20 (43.5) | 17 (35.4) | |
| Irregular | 2 (4.3) | 8 (16.7) | 0.045 |
| Fusional | 0 (0) | 8 (16.7) | 0.006 |
| Border | |||
| Distinct | 34 (73.9) | 29 (60.4) | |
| Indistinct | 12 (26.1) | 13 (27.1) | |
| Infiltrative signs | 0 (0) | 6 (12.5) | 0.027 |
| Echogenicity | |||
| Hypoechoic | 40 (87.0) | 38 (79.1) | |
| Slightly hyperechoic | 6 (13.0) | 2 (4.2) | |
| Slightly hyperechoic | 0 | 8 (16.6) | 0.006 |
Compared with the operative and pathological findings, the accuracy of transabdominal US for T1, T2, T3, T4 was 55.6%, 75.0%, 87.3%, 71.1%, respectively, and the overall accuracy was 77.6% (Table 1).
Although the accuracy of transabdominal US in staging of T1 carcinoma was low (55.6%), T2, T3, and T4 staging results were compared favorably with those of EUS T staging. Lim et al[15] reported the accuracy of staging in early-stage carcinoma was 66.7% (10 of 15 patients) with transabdominal US. In the study of Segura et al[16], the accuracy of transabdominal US for T1, T2, T3, T4 was 100%, 50%, 87%, 81%, respectively. Due to the small number of patients with early stage carcinoma (2 patients), the results of T1 staging required further investigation.
In our study, the accuracy for T3 carcinomas was the highest (87.3%) among the four groups with different depths of infiltration. The normal serosal layer was a thin and smooth hyperechoic band sonographically. When tumor invaded into the serosal layer, this band was interrupted or disappeared, which could be assessed easily with transabdominal US.
In our study, the accuracy for T1 carcinomas was most unfavorable (55.6%) because of overstaging in 4 patients, of which 3 had ulcerative carcinoma. Similarly, misdiagnosis was made in 4 patients with T2 carcinoma due to overstaging as a T3 carcinoma, and these 4 patients also had ulcerative carcinoma. Previous studies showed that the hyperechoic submucosal layer became hypoechoic because of inflammation or edema around the tumor, which was similar to echogenicity of muscularis propria and tumor. It was difficult to differentiate 3 layers with transabdominal US[19], so T1 carcinoma might be overstaged as T2 carcinoma (tumor invading muscularis propria). On the other hand, the ulcer in gastric cancer may cause gastric wall fibrosis and scar, which lead to wall thickening, loss of wall layers, and even interruption or disappearance of serosal layer, therefore, overstaging as T3 carcinoma may occur in T1 or T2 carcinoma (Figure 2). Difficulties in differentiation between tumors and peritumor inflammation or scar may be an important source of overstaging[5,6,15,20-23].
As shown in Table 1, misdiagnosis was made in T1 and T2 carcinomas because of overstaging. On the contrary, misdiagnosis was made in T3 and T4 carcinomas because of understaging. The possible reasons for understaging were as follows: (1) The procedure did not practise adequately, because transabdominal US was technically difficult to perform and may fail to guarantee a correct diagnosis for tumors locating in gastric fundus, greater curvature or cardia[15]. Tumors in 8 of 20 understaged patients located in the above locations, respectively. (2) Location of involved organs also affected the diagnosis, because it was difficult to clearly visualize hilum of spleen, diaphragm, and the tail of pancreas. Invasions of the tail of pancreas (4 patients), diaphragm (2 patients), spleen and hilum of spleen (2 patients) were not detected with transabdominal US. (3) Microscopic tumor invasion undetected with transabdominal US was another cause of understaging[6], which occurred in 2 patients with T3 carcinoma.
The fact that gastric cancer invaded into adjacent organs would decrease surgical resectability, so it is important to assess preoperatively if organs are invaded by gastric cancer.
In our study, the followings were taken as the diagnostic criteria for gastric cancer invading into organs: interruption of the serosal layer of gastric wall, indistinguishable from adjacent organs, tumor and organs moving synchronously slow or no moving as breath changed.
Pancreas was the most common organ invaded by tumor, the detection rate in this study was 77.4%. In 3 of 7 patients undetected by transabdominal US, pancreas was invaded slightly, and the boundary between pancreas and tumor seemed to exist on sonogram, thus leading to understaging. In the remaining 4 patients, the tail of pancreas was invaded, which was not detected by transabdominal US due to interference of bowel gas and ribs.
The detection rate for liver invasion was higher (71.4%). Tumors invading liver located in anterior wall of stomach or lesser curvature and were close to the liver. The relationship between tumor and liver could be visualized clearly by using ultrasonographic beam through liver without interference of bowel gases.
Transverse colon was invaded in three patients, of them, correct diagnosis was made in 1 patient because complete circumference of transverse colon was invaded and marked tumor was detected. In two others undetected by transabdominal US, transverse colon was slightly invaded and bowel gases interfered severely. Correct diagnosis was made preoperatively in 1 of 3 patients with spleen and its hilum invasion. None was diagnosed preoperatively for diaphragm invasion. Spleen and its hilum, and diaphragm could not be observed completely with transabdominal US, leading to the low detection rate.
Correct diagnosis was made preoperatively in 4 of 9 patients with duodenum invasion. It was necessary to fill duodenum by drinking water for the assessment of duodenum. Because tumor caused gastric lumen obstruction, duodenum was not filled adequately and visualized clearly, leading to misdiagnosis of duodenum invasion.
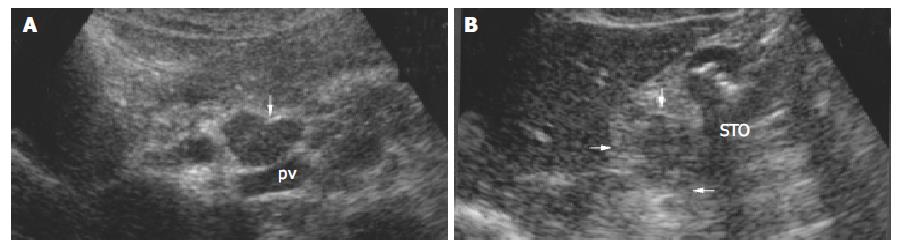
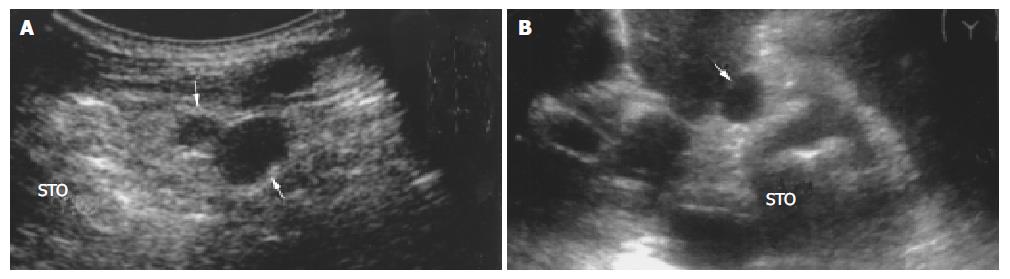
The sensitivity of transabdominal US for lymph node metastasis was 77.6%, and the false positive rate was 13.2% (14 of 106 patients). The results showed transabdominal US did not permit the differentiation between benign and (metastatic) malignant lymph nodes in gastric cancer (Figure 4, Figure 5).
Benign swollen lymph nodes due to immune reaction in patients with tumor are misdiagnosed easily as malignant lymph nodes. According to the analysis of sonographic features of lymph nodes (Table 4), benign lymph nodes were smaller than malignant lymph nodes in length and width (P = 0.000, 0.005). From Table 5, if the length ≥ 1.1 cm or 1.6 cm was defined as diagnostic criteria of malignant lymph nodes, the sensitivity was 81.3% and 47.9%, respectively, and the specificity was 56.5% and 76.1%, respectively. Hence the criteria could not differentiate benign from malignant lymph nodes. If the length ≥ 2.1 cm or the width ≥ 1.6 cm was defined as diagnostic criteria of malignant lymph nodes, the sensitivity was 33.3% and 29.2%, respectively, and the specificity was 95.7% and 93.5%, respectively. This result showed that lymph node with a length ≥ 2.1 cm or width ≥ 1.6 cm increased significantly the probability of metastasis. From the literature and our study, although benign lymph nodes were smaller than malignant lymph nodes, it is clear that there was a considerable overlap in size between benign and malignant lymph nodes; therefore, the value of lymph node size is limited for discriminating benign lymph nodes from malignant lymph node[18,24,25].
| Criterion | Sensitivity (%) | Specificity (%) |
| Length ≥ 1.1cm | 81.3 (39/48) | 56.5 (26/46) |
| ≥1.6 cm | 47.9 (23/48) | 76.1 (35/46) |
| ≥2.1 cm | 33.3 (15/48) | 95.7 (44/46) |
| Width ≥ 1.1 cm | 50.0 (24/48) | 84.8 (39/46) |
| ≥ 1.6 cm | 29.2 (14/48) | 93.5 (43/46) |
| Irregular | 16.7 (8/48) | 95.7 (44/46) |
| Fusional | 16.7 (8/48) | 100 (46/46) |
| Infiltrative signs | 12.5 (6/48) | 100 (46/46) |
| Inhomogenous | 16.7 (8/48) | 100 (46/46) |
In our study (Table 2), benign and malignant lymph nodes in round shape were encountered most commonly (Figure 4, Figure 5) and spindle and ovoid lymph nodes were also seen , there was no significant difference between two kinds of lymph nodes. Malignant lymph nodes in irregular shape were mostly seen, and all fusional-shaped lymph nodes were malignant (Figure 3), there was a significant difference between benign and malignant lymph nodes (P = 0.045, 0.006). It was reported that abdominal malignant lymph nodes were round or ovoid in shape, conversely, benign lymph nodes in people who were healthy or affected by benign disease were spindle or flat in shape[9,18,24,26-30]. However in our study, both benign and malignant lymph nodes were mostly round in shape, and round shape did not allow the correct differentiation between benign and malignant lymph nodes. Immune reaction caused by tumor was different from that caused by benign diseases such as hepatitis or entities, which may be a cause for difficulties in differentiation between benign and malignant lymph nodes.
In our study the majority of benign and malignant lymph nodes had a distinct border (Figure 5), without statistical difference between them (Table 4). “Infiltrative signs” of lymph nodes were seen only in malignant lymph nodes (P = 0.027), which were typical and helpful for diagnosing malignant lymph nodes. Heintz et al[31] reported that both benign and malignant lymph nodes had an indistinct or distinct border by studying surgical resection specimens of patients with esophageal and gastric carcinomas. The study reported by Akahoshi et al[29,32] showed that malignant lymph nodes had a distinct border.
Table 4 shows that both benign and malignant lymph nodes were hypoechoic mostly (Figure 3, Figure 4, Figure 5), and the small number of lymph nodes was hyperechoic. There was no difference between benign and malignant lymph nodes. Inhomogenous echogenicity was seen only in malignant lymph nodes, there was a significant difference between benign and malignant lymph nodes (P = 0.006) and majority of inhomogenous lymph nodes were 2 cm greater in length. Contradictory data were published for echogenicity of lymph nodes. Heintz et al[31] stressed that echogenicity did not allow the correct differentiation between benign and malignant lymph nodes. Other authors[24,33] reported that most malignant lymph nodes were hypoechoic, and most benign lymph nodes were isoechoic or slightly hyperechoic. The different results might result from different patients studied.
In our study, the following 4 sonographic features were taken as the diagnostic criteria of malignant lymph nodes (Table 5): irregular shape, fusional shape, infiltrative signs, and inhomogenous echo. The sensitivity of the criteria was lower than 20 percent, and the specificity was higher than 90 percent. One of these 4 features was helpful to suggest malignant, however the similar sonographic features were observed in abdominal tuberculosis. Differential diagnosis was made on the basis of medical history.
Swollen lymph nodes were found pathologically in 106 patients. But no swollen lymph nodes were detected with transabdominal US in 40 of 106 patients, and lymph nodes metastases were confirmed pathologically in 15 of these 40 patients. Missing lymph nodes were associated with the size of lymph nodes mainly. Lymph nodes less than 5 mm in size were usually undetectable with transabdominal US. In addition, obesity and bowel gas were unfavorable for detection of lymph nodes.
In conclusion, transabdominal US is useful for preoperative staging of gastric cancer, especially for assessment of advanced gastric cancer invasion. Although it is difficult to differentiate benign from malignant lymph nodes, typical sonographic features might contribute to diagnosis.
| 1. | Sipponen P, Järvi O, Kekki M, Siurala M. Decreased incidences of intestinal and diffuse types of gastric carcinoma in Finland during a 20-year period. Scand J Gastroenterol. 1987;22:865-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Silverberg E. Cancer statistics, 1980. CA Cancer J Clin. 1980;30:23-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Gelfand DW, Ott DJ. Single- vs. double-contrast gastrointestinal studies: critical analysis of reported statistics. AJR Am J Roentgenol. 1981;137:523-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | White RM, Levine MS, Enterline HT, Laufer I. Early gastric cancer. Recent experience. Radiology. 1985;155:25-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Xi WD, Zhao C, Ren GS. Endoscopic ultrasonography in preoperative staging of gastric cancer: determination of tumor invasion depth, nodal involvement and surgical resectability. World J Gastroenterol. 2003;9:254-257. [PubMed] |
| 6. | Wang JY, Hsieh JS, Huang YS, Huang CJ, Hou MF, Huang TJ. Endoscopic ultrasonography for preoperative locoregional staging and assessment of resectability in gastric cancer. Clin Imaging. 1998;22:355-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Rösch T. Endosonographic staging of gastric cancer: a review of literature results. Gastrointest Endosc Clin N Am. 1995;5:549-557. [PubMed] |
| 8. | Ohashi S, Nakazawa S, Yoshino J. Endoscopic ultrasonography in the assessment of invasive gastric cancer. Scand J Gastroenterol. 1989;24:1039-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Botet JF, Lightdale CJ, Zauber AG, Gerdes H, Winawer SJ, Urmacher C, Brennan MF. Preoperative staging of gastric cancer: comparison of endoscopic US and dynamic CT. Radiology. 1991;181:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 187] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Bolondi L, Casanova P, Caletti GC, Grigioni W, Zani L, Barbara L. Primary gastric lymphoma versus gastric carcinoma: endoscopic US evaluation. Radiology. 1987;165:821-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Puylaert JB. Acute appendicitis: US evaluation using graded compression. Radiology. 1986;158:355-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 402] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 12. | Kimmey MB, Martin RW, Haggitt RC, Wang KY, Franklin DW, Silverstein FE. Histologic correlates of gastrointestinal ultrasound images. Gastroenterology. 1989;96:433-441. [PubMed] |
| 13. | Wiersema MJ, Wiersema LM. High-resolution 25-megahertz ultrasonography of the gastrointestinal wall: histologic correlates. Gastrointest Endosc. 1993;39:499-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Lim JH, Jeong YM. Sonography of the stomach: an in vitro study to determine the anatomic cause of inner hyperechoic and hypoechoic layers of the gastric wall. AJR Am J Roentgenol. 1994;162:335-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Lim JH, Ko YT, Lee DH. Transabdominal US staging of gastric cancer. Abdom Imaging. 1994;19:527-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Segura JM, Olveira A, Conde P, Erdozain JC, Suárez J. Hydrogastric sonography in the preoperative staging of gastric cancer. J Clin Ultrasound. 1999;27:499-504. [PubMed] [DOI] [Full Text] |
| 17. | Sobin LH, Hermanek P, Hutter RV. TNM classification of malignant tumors. A comparison between the new (1987) and the old editions. Cancer. 1988;61:2310-2314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Smeets AJ, Zonderland HM, van der Voorde F, Laméris JS. Evaluation of abdominal lymph nodes by ultrasound. J Ultrasound Med. 1990;9:325-331. [PubMed] |
| 19. | Tio TL, Coene PP, Schouwink MH, Tytgat GN. Esophagogastric carcinoma: preoperative TNM classification with endosonography. Radiology. 1989;173:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 107] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Grimm H, Binmoeller KF, Hamper K, Koch J, Henne-Bruns D, Soehendra N. Endosonography for preoperative locoregional staging of esophageal and gastric cancer. Endoscopy. 1993;25:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 110] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Dittler HJ, Siewert JR. Role of endoscopic ultrasonography in gastric carcinoma. Endoscopy. 1993;25:162-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 76] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Tio TL, Coene PP, Luiken GJ, Tytgat GN. Endosonography in the clinical staging of esophagogastric carcinoma. Gastrointest Endosc. 1990;36:S2-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 59] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Yanai H, Matsumoto Y, Harada T, Nishiaki M, Tokiyama H, Shigemitsu T, Tada M, Okita K. Endoscopic ultrasonography and endoscopy for staging depth of invasion in early gastric cancer: a pilot study. Gastrointest Endosc. 1997;46:212-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 112] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 24. | Gimondo P, Mirk P, Messina G, Pizzi C. Abdominal lymphadenopathy in benign diseases: sonographic detection and clinical significance. J Ultrasound Med. 1996;15:353-39; quiz 353-39;. [PubMed] |
| 25. | Dorfman RE, Alpern MB, Gross BH, Sandler MA. Upper abdominal lymph nodes: criteria for normal size determined with CT. Radiology. 1991;180:319-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 233] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | Sutton RT, Reading CC, Charboneau JW, James EM, Grant CS, Hay ID. US-guided biopsy of neck masses in postoperative management of patients with thyroid cancer. Radiology. 1988;168:769-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 80] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Sakai F, Kiyono K, Sone S, Kondo Y, Oguchi M, Watanabe T, Sakai Y, Imai Y, Takeda S, Yamamoto K. Ultrasonic evaluation of cervical metastatic lymphadenopathy. J Ultrasound Med. 1988;7:305-310. [PubMed] |
| 28. | Vassallo P, Wernecke K, Roos N, Peters PE. Differentiation of benign from malignant superficial lymphadenopathy: the role of high-resolution US. Radiology. 1992;183:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 351] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 29. | Akahoshi K, Misawa T, Fujishima H, Chijiiwa Y, Nawata H. Regional lymph node metastasis in gastric cancer: evaluation with endoscopic US. Radiology. 1992;182:559-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Subramanyam BR, Balthazar EJ, Horii SC, Hilton S. Abdominal lymphadenopathy in intravenous drug addicts: sonographic features and clinical significance. AJR Am J Roentgenol. 1985;144:917-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Heintz A, Mildenberger P, Georg M, Braunstein S, Junginger T. Endoscopic ultrasonography in the diagnosis of regional lymph nodes in esophageal and gastric cancer--results of studies in vitro. Endoscopy. 1993;25:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | François E, Peroux J, Mouroux J, Chazalle M, Hastier P, Ferrero J, Simon J, Bourry J. Preoperative endosonographic staging of cancer of the cardia. Abdom Imaging. 1996;21:483-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Metreweli C, Ward SC. Ultrasound demonstration of lymph nodes in the hepatoduodenal ligament ('Daisy Chain nodes') in normal subjects. Clin Radiol. 1995;50:99-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
Edited by Wang XL and Chen WW Proofread by Xu FM