Published online Nov 1, 2004. doi: 10.3748/wjg.v10.i21.3208
Revised: December 1, 2003
Accepted: December 8, 2003
Published online: November 1, 2004
AIM: To explore the influence of hepatic glucose production on acute insulin resistance induced by a lipid infusion in awake rats.
METHODS: A hyperinsulinaemic-euglycaemic clamp was established in awake chronically catheterized rats. Two groups of rats were studied either with a 4-h intraarterial infusion of lipid/heparin or saline. Insulin-mediated peripheral and hepatic glucose metabolism was assessed by hyperinsulinaemic-euglycaemic clamp combined with [3-3H]-glucose infusion.
RESULTS: During hyperinsulinaemic-euglycaemic clamp, there was a significant increase in plasma free fatty acid (FFA, from 741.9 ± 50.6 to 2346.4 ± 238.5 μmol/L, P < 0.01) in lipid-infused group. The glucose infusion rates (GIR) in the lipid infusion rats, compared to control rats, were significantly reduced (200-240 min average: Lipid infusion; 12.6 ± 1.5 vs control; 34.0 ± 1.6 mg/kg.min, P < 0.01), declining to - 35% of the corresponding control values during the last time of the clamp (240 min: Lipid infusion; 12.0 ± 1.9 vs control; 34.7 ± 1.7 mg/kg·min, P < 0.0001). At the end of clamp study, the hepatic glucose production (HGP) in control rats was significantly suppressed (88%) from 19.0 ± 4.5 (basal) to 2.3 ± 0.9 mg/kg.min (P < 0.01). The suppressive effect of insulin on HGP was significantly blunted in the lipid-infused rats (200-240 min: From 18.7 ± 3.0 to 23.2 ± 3.1 mg/kg·min (P < 0.05). The rate of glucose disappearance (GRd) was a slight decrease in the lipid-infused rats compared with controls during the clamp.
CONCLUSION: These data suggest that lipid infusion could induces suppression of hepatic glucose production, impairs the abilities of insulin to suppress lipolysis and mediate glucose utilization in peripheral tissue. Therefore, we conclude that lipid-infusion induces an acute insulin resistance in vivo.
- Citation: Li L, Yang GY. Effect of hepatic glucose production on acute insulin resistance induced by lipid-infusion in awake rats. World J Gastroenterol 2004; 10(21): 3208-3211
- URL: https://www.wjgnet.com/1007-9327/full/v10/i21/3208.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i21.3208
Insulin resistance plays a primary role in the development of type 2 diabetes and is a feature of other disorders including obesity, dyslipidemias, hypertension, and cardiovascular disease[1]. The mechanism underlying the occurrence of insulin resistance is unknown but may be related to alterations in lipid metabolism[2]. More than 30 years ago, Randle and colleagues demonstrated that free fatty acids (FFA) competed with glucose for substrate oxidation in isolated rat heart and diaphragm muscle preparations and speculated that increased fat oxidation might cause insulin resistance associated with diabetes and obesity[3,4]. Subsequently, some studies have emphasized that, while an increase in circulating FFA during insulin clamp studies could promptly decrease the rate of carbohydrate oxidation, defective glucose uptake which could be detected 3-4 h after lipid infusion in humans[5,6]. Roden and Dresner have revealed that lipid/heparin infusions could increase plasma FFA levels, inhibit whole-body glucose disposal during hyper- and euglycemic-hyperinsulinemia and insulin-dependent glucose uptake by human forearm tissues in vivo, and also found that acute elevations in plasma fatty acids in humans resulted in decreased glucose transport activity, as reflected by decreased concentrations of intracellular glucose 6-phosphate and glucose[7,8]. Thus, it is possible that chronic elevation of endogenous FFAs contributes to insulin resistance in many pathophysiologic conditions in humans. Acute elevations in plasma FFA levels during a triglyceride emulsion infusion have also been shown to impaire insulin-mediated glucose uptake and to inhibit hepatic glucose production (HGP) in rats[9].
In the current study, we used a triglyceride and cholesterolester emulsion infusion in combination with hyperinsulinemic-euglycemic clamps to assess the impact of elevated FFA levels on HPG and overall insulin action.
A total of 24 Male Sprague-Dawley rats weighing 250-300 g were housed in individual cages and subjected to an environmentally controlled room with a 12-h light/dark cycle, where they had free access to standard rat chow and water. Five to 7 d before the in vivo study, rats were anesthetized with an intraperitoneal injection of pentobarbital (50 mg/kg body mass). A silastic catheter (I.D. = 0.02 in ) was inserted into the right internal jugular vein and extended to the level of the right atrium. The catheter for carotid artery was constructed with a short (25 mm) segment of polyethylene tubing (PE-10), connected to a 10-cm length of PE-50 by heating in a flame. The smaller end was advanced through the left carotid artery until its tip reached the aortic arch. The free ends of the catheters were attached to the long segments of steel tubing and tunneled subcutaneously around the side to the back of the neck where they were exteriorized through a skin incision and then securely anchored to the skin by a standard wounded clip. At the end of the procedure, catheters were flushed with 300 μL isotonic saline containing heparin (20 U/mL) and ampicillin (5 mg/mL) and then filled with a viscous solution of heparin (300 U) and 800 g/L polyvinylpyrollidone (PVP-10, Fisher, NJ) to prevent refluxing of blood into the catheter lumen.
Animals were allowed at least five days to recover from the effects of surgery. All studies were conducted in the morning following a 12 to 14-h overnight fast. Throughout the study, the rats were allowed to move freely within the confines of a cage. One hour before clamping the venous and arterial lines were filled with a 9 g/L NaCl solution containing 10 IU/mL heparin. Three double lumen swivels, allowing separate fluid infusions, were connected to three peristaltic pumps. One arterial line was used for the infusion of a 250 g/L glucose solution at a variable rate and the other line was used for infusion of a mixture of (3-3H)-glucose (Amersham Inc, USA), insulin and 150 mL/L lipid emulsion/heparin (20 U/mL). The venous blood sampling tube allowed frequent sampling and repletion of blood loss by means of fresh whole blood obtained from littermates.
At the start of euglycaemic clamp, continuous infusions of isotonic saline (control group, n = 12) and lipid emulsion with heparin (lipid group, n = 12) were maintained for 4 h at a rate 1.5 mL/h during prolonged euglycemic-hyperinsulinemic clamp studies. At t = 60 min, a bolus (6 μCi) and continuous infusion (0.2 μCi/min ) of (3-3H) glucose were initiated and continued throughout 3 h study. At t = 120 min, continuous infusions of insulin (4.8 mU/kg·min) and 250 g/L glucose were maintained for 2 h, 250 g/L glucose was adjusted every 5-10 min, maintaining basal plasma glucose concentrations (-5 mmol/L) during the insulin clamp studies. At t = 0, 120, 200, 220, 230 and 240 min, blood samples were collected for determination of plasma glucose, insulin, free fatty acid (FFA) and specific activity of tritiated glucose.
A separate set of 240 min lasting control clamp experiments without lipid infusion were performed to investigate the self-amplifying effect of long-term clamping on insulin-mediated glucose metabolism, since was glucose metabolism was not constant during a 240 min study[10]. The experimental procedure was identical to the lipid infusion clamps.
Plasma insulin was measured by radioimmunoassay (RIA) using rat insulin as standard (Linco Research, Inc. MO). Inter- and intra-assay variations of the insulin assay were 5.8% and 6.5%, respectively. Enzymatic colorimetric kits were used to determine plasma concentration of FFA ( Wako Chemicals, Inc. VA). The inter- and intra-assay variations were 3.6% and 4.2%, respectively, during measurement of plasma FFA. Plasma for [3-3H]-glucose radioactivity (150 μL) was deproteinized by barium hydroxide-zinc sulphate, the supernatant was evaporated to dryness at 60 °C to eliminate tritiated water and counted for 10 min in a beta scintillation counter.
The rate of exogenous infused glucose to maintain euglycaemia during the steady-state period (from t = 180-240 min) was used for the assessment of insulin action. All calculations were carried out in this period when the total amount of glucose taken up by all tissues of the body was equal to the input of glucose into the body. During this steady-state, when the rate of glucose appearance (GRa) was equal to the rate of glucose disappearance (GRd), the glucose turnover rate, which equaled to GRa and GRd in mg/min, was calculated by dividing the [3-3H]-glucose infusion rate (dpm/mg) by the steady-state value of glucose specific activity (dpm/mg). Under these conditions, the glucose turnover rate was equal to the sum of the rates of exogenous infused glucose and of hepatic glucose production (HGP). From this equation the rate of HGP was calculated. Since urinary glucose loss was not present, peripheral glucose uptake (PGU) was taken as glucose turnover rate which equaled to exogenous glucose infusion rate plus rate of HGP.
Data were presented with mean ± SD. Comparisons between groups were made by the two-tailed Student’s t test. All statistical analyses were performed using SPSS.
There were no differences in the mean body mass between control and lipid-infusion rats. Basic plasma concentrations of glucose, insulin, and free fatty acids (FFA) were similar in the two groups (Table 1).
| Group | Control | Lipid-infusion |
| Body mass (g) | 279 ± 19 | 286 ± 17 |
| Fasting blood glucose (mmol/L) | 5.2 ± 0.1 | 5.1 ± 0.2 |
| Fasting plasma insulin (mU/L) | 30.3 ± 2.4 | 27.9 ± 2.2 |
| Fasting free fatty acids (μmol/L) | 672.5 ± 92.2 | 741.9 ± 50.6 |
As expected, following surgery catheterized animals lost significant weight during the initial 24-h period, averaged 18 ± 3 g. Following this catabolic stage they appeared well and normally active. Food intake was qualitatively normal, and average daily weight gain (-5g) closely resembled that of normal littermates that did not undergo surgery.
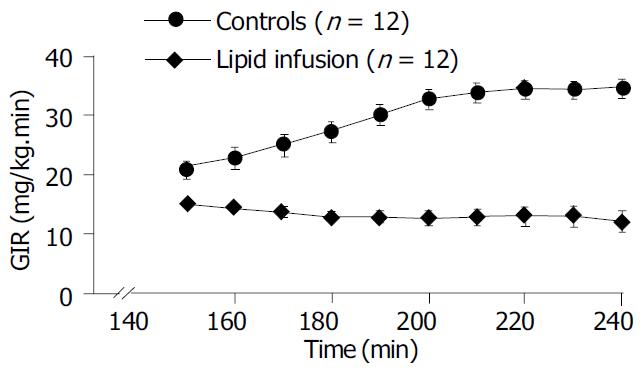
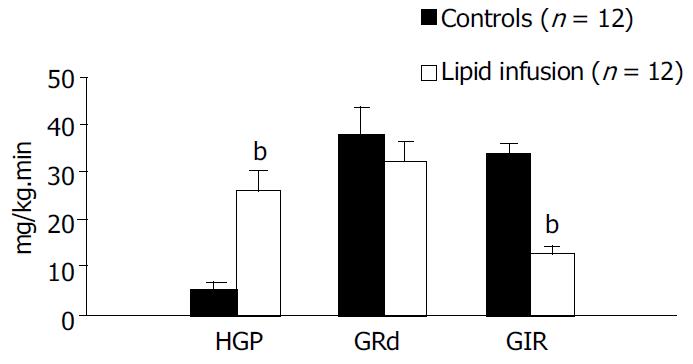
A lipid infusion of 4 h at a rate of 1 mL/h was used to examine the effect on plasma insulin, FFA, peripheral glucose uptake and hepatic glucose production. During the euglycemic-hyperinsulinemic clamps blood glucose concentrations remained constant compared to the basal levels and were not different between control and lipid infusion studies. Plasma insulin concentrations increased similarly to -100 mU/L in both studies (Table 2). The coefficients of variation in plasma glucose and insulin levels were 4.8% and 7.6 % , respectively, in all studies. In the control study the plasma concentration of FFAs dropped by -65% once the euglycemic clamp was started, but it increased approximately fourfold (from 741.9 ± 50.6 to 2346.4 ± 238.5 μmol/L, P < 0.01) within 120 min of hyperinsulinemic clamp in the lipid - infused group (Table 2). The time course of the glucose infusion rate (GIR) during insulin clamp is shown in Figure 1. The GIR in lipid infusion rats, compared to control rats, was significantly reduced (200-240 min average: Lipid infusion; 12.6 ± 1.5 vs control; 34.0 ± 1.6 mg/kg·min, P < 0.01, Table 2 and Figure 2), declining to -35% of the corresponding control values during the last time of the clamp (240 min: Lipid infusion; 12.0 ± 1.9 vs control; 34.7 ± 1.7 mg/kg·min, P < 0.0001, Figure 1). After a 14-h fast there was no significant difference in HGP between the two groups (19.0 ± 4.5 vs 18.7 ± 3.0 mg/kg·min in control and lipid-infused rats, respectively). At the end of hyperinsulinemic-euglycemic clamp study, the HGP in control rats was significantly suppressed (88%) from 19.0 ± 4.5 (basal) to 2.3 ± 0.9 mg/kg·min (P < 0.01, Table 2). The suppressive effect of insulin on HGP was significantly blunted in lipid-infused rats (180-240 min: Lipid infusion; from 18.7 ± 3.0 to 23.2 ± 3.1 mg/kg·min P < 0.05, Table 2). The time courses of HGP for controls and lipid-infused rats are shown in Table 2. During the clamp, the GRd was significant increased compared with basal values. Although the GRd had no significant difference between the two groups, there was a slight decrease in lipid-infused rats compared with controls (Table 2). Figure 2 shows the average values of HGP, GRd and GIR during the clamp in control and lipid-infused rats.
| Group | Basal | Clamping time (min) | ||||
| 120 | 200 | 220 | 230 | 240 | ||
| Glucose (mmol/L) | ||||||
| Control (n = 12) | 5.2 ± 0.1 | 5.3 ± 0.1 | 5.0 ± 0.2 | 5.2 ± 0.1 | 5.2 ± 0.2 | 5.4 ± 0.2 |
| Lipid (n = 12) | 5.1 ± 0.2 | 5.3 ± 0.2 | 5.2 ± 0.2 | 5.3 ± 0.2 | 5.5 ± 0.2 | 5.5 ± 0.2 |
| FFA (μmol/L) | ||||||
| Control (n = 12) | 672.5 ± 92.2 | 642.3 ± 104.8 | 240.6 ± 20.9 | 221.3 ± 27.4 | 201.8 ± 29.8 | 183.1 ± 21.4 |
| Lipid (n = 12) | 741.9 ± 50.6 | 2807.2 ± 348.8bd | 3086.9 ± 495.2bd | 2518.8 ± 416.7bd | 2241.2 ± 431.0bd | 2346.4 ± 238.5bd |
| Insulin (mU/L) | ||||||
| Control (n = 12) | 30.3 ± 2.4 | 34.7 ± 6.4 | 89.5 ± 7.9 | 90.7 ± 7.4 | 88.3 ± 5.9 | 101.3 ± 6.3 |
| Lipid (n = 12) | 27.9 ± 2.2 | 31.4 ± 3.3 | 84.2 ± 6.7 | 93.3 ± 8.9 | 103.5 ± 16.3 | 104.5 ± 14.8 |
| GIR (mg/kg· min) | ||||||
| Controls (n = 12) | 0 | 32.8 ± 1.7 | 34.4 ± 1.6 | 34.4 ± 1.6 | 34.7 ± 1.7 | |
| Lipid (n = 12) | 0 | 12.7 ± 1.3b | 12.9 ± 1.6b | 12.9 ± 1.8b | 12.0 ± 1.9b | |
| GRd (mg/kg·min) | ||||||
| Controls (n = 12) | 19.0 ± 4.5 | 43.1 ± 6.1d | 40.1 ± 6.7d | 35.5 ± 6.8d | 33.2 ± 3.2d | |
| Lipid (n = 12) | 18.7 ± 3.0 | 36.3 ± 3.1d | 38.3 ± 4.4d | 32.4 ± 4.5d | 35.1 ± 3.9d | |
| HGP (mg/kg·min) | ||||||
| Controls (n = 12) | 19.0 ± 4.5 | 14.2 ± 4.9c | 10.2 ± 4.4d | 3.1 ± 1.9d | 2.3 ± 0.9d | |
| Lipid (n = 12) | 18.7 ± 3.0 | 23.4 ± 4.3bc | 25.3 ± 3.7bd | 21.5 ± 3.5b | 23.2 ± 3.1bd | |
Obesity is associated with insulin resistance and hyperinsulinemia, two important cardiovascular risk factors[11]. What remains uncertain is how obesity produces insulin resistance and hyperinsulinemia. It has recently become clear, however, that FFA plays a pivotal role in this process. Although there are a number of studies on this subject, the precise mechanisms of FFA effect on insulin action are not completely understood. In the present study we examined the effect of a 4-h lipid infusion on in vivo insulin action in conscious rats, by the hyperinsulinaemic-euglycaemic clamp technique, in combination with a continuous infusion of [3-3H]-glucose. This method is considered to be the most suitable for the measurement of in vivo insulin. In our hyperinsulinemic clamping, insulin infusion increased plasma insulin levels to an approximate three-fold over basal insulin level, whereas blood glucose was clamped at approximately 5.3 mmol/L in the control and lipid infusion groups. Plasma FFA concentrations were suppressed by approximately -65% during clamps in the control group. But in lipid infusion rats, the FFA levels had a rapid increase more than 3.8-fold over basal levels and the increase was maintained to the end of hyperinsulinemic clamp, suggesting that lipid infusion impaired the antilipolytic action of insulin and promoted the release of fatty acids from adipocytes or infused lipid. It is widely known that elevated FFA levels could exert a deleterious effect on insulin’s overall actions, and this has been demonstrated in both animals and humans[12]. The mechanisms underlying FFA-induced insulin resistance are not very clear, but elevated plasma levels of FFA produced at least two distinct biochemical defects: Inhibition of insulin stimulated glucose transport and/or phosphorylation, and inhibition of muscle glycogen systhase activity[13].
In the attempt to better our understanding of the pathophysiology of lipid-induced insulin resistance, we examined the effect of lipid infusions on HGP. We found that HGP was suppressed by -88% in the controls during hyperinsulinaemic-euglycaemic clamp, suggesting the impact of insulin on the suppression of endogenous glucose production. However, HGP was not significantly suppressed in lipid-infused rats. Thus, lipid infusion elevated the levels of circulating FFA and elevated FFA levels interfered with insulin’s ability to inhibit hepatic glucose production. The increased hepatic glucose production in response to lipid infusion suggested that an experimental elevation of circulating FFA levels could lead to hepatic insulin resistance. On the other hand, during insulin clamp glucose infusion rates (GIR), compared to control rats, were significantly reduced to -35% of the corresponding control values during the last time of the clamp. At clamping steady state, peripheral glucose uptake was equal to the sum of the rates of exogenous infused glucose (GIR) and hepatic glucose production (HGP). If HGP was completely suppressed, peripheral glucose uptake would equal to GIR (in controls). But, HGP was not significantly suppressed in lipid-infused rats, so peripheral glucose uptake was equal to GRa, which equaled to HGP plus GIR. Total glucose uptake is the sum of glucose removal by insulin-dependent as well as insulin-independent tissues. In the present study we found there was a slight decrease in GRa in lipid infusion groups, although the data did not reach statistical significance. Nonetheless, the trend is obvious since the brain and splanchnic tissues use glucose in an insulin-independent manner, roughly 750 g/L of total glucose utilization is considered to be insulin-independent in fasting condition. Thus, this might indicate that 4 h of lipid infusion induced a partial defect in insulin-stimulated peripheral glucose uptake, consistent with previous in vivo studies by Kim et al[14]. Considering the absence of HGP-suppressing effect of insulin under lipid infusion condition, we concluded that an experimental elevation of circulating FFA levels by lipid/heparin infusions could lead to peripheral and hepatic insulin resistance. The mechanism of lipid-induced insulin resistance remains poorly understood and may involve different IRS-1-associated PI3-kinase activation[15], and the activity of Iκ B kinase-β (Ikk-β , a known serine kinase)[14,16].
In summary, the current studies showed that infusion of lipid emulsions with heparin to acutely raise plasma fatty acid concentrations could impaire the ability of insulin to stimulate overall body glucose disposal and also interfered with insulin’s ability to inhibit hepatic glucose production. Thus, we propose that a sustained increase in circulating FFA causes a hepatic insulin resistance, and may lead to a partial defect in insulin-stimulated peripheral glucose uptake, which can be attributed to lipotoxic effect on insulin action.
| 1. | Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5710] [Cited by in RCA: 5963] [Article Influence: 156.9] [Reference Citation Analysis (0)] |
| 2. | Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 781] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 3. | Randle PJ, Garland PB, Newsholme EA, Hales CN. The glucose fatty acid cycle in obesity and maturity onset diabetes mellitus. Ann N Y Acad Sci. 1965;131:324-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 164] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3466] [Cited by in RCA: 3382] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 5. | Boden G, Jadali F, White J, Liang Y, Mozzoli M, Chen X, Coleman E, Smith C. Effects of fat on insulin-stimulated carbohydrate metabolism in normal men. J Clin Invest. 1991;88:960-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 350] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 6. | Kelley DE, Mokan M, Simoneau JA, Mandarino LJ. Interaction between glucose and free fatty acid metabolism in human skeletal muscle. J Clin Invest. 1993;92:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 331] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996;97:2859-2865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 1029] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 8. | Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103:253-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 903] [Cited by in RCA: 862] [Article Influence: 31.9] [Reference Citation Analysis (7)] |
| 9. | Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, Goodyear LJ, Kraegen EW, White MF, Shulman GI. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48:1270-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 898] [Cited by in RCA: 849] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 10. | Koopmans SJ, van Mansfeld AD, Jansz HS, Krans HM, Radder JK, Frölich M, de Boer SF, Kreutter DK, Andrews GC, Maassen JA. Amylin-induced in vivo insulin resistance in conscious rats: the liver is more sensitive to amylin than peripheral tissues. Diabetologia. 1991;34:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Fontbonne AM, Eschwège EM. Insulin and cardiovascular disease. Paris Prospective Study. Diabetes Care. 1991;14:461-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 133] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Hevener AL, Reichart D, Janez A, Olefsky J. Thiazolidinedione treatment prevents free fatty acid-induced insulin resistance in male wistar rats. Diabetes. 2001;50:2316-2322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Boden G. Free fatty acids (FFA), a link between obesity and insulin resistance. Front Biosci. 1998;3:d169-d175. [PubMed] |
| 14. | Kim JK, Kim YJ, Fillmore JJ, Chen Y, Moore I, Lee J, Yuan M, Li ZW, Karin M, Perret P. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest. 2001;108:437-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 517] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 15. | Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230-50236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1102] [Cited by in RCA: 1129] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 16. | Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-β. Nature. 1998;396:77-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1219] [Cited by in RCA: 1254] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
Edited by Zhang JZ and Wang XL Proofread by Xu FM