Published online Nov 1, 2004. doi: 10.3748/wjg.v10.i21.3151
Revised: January 8, 2004
Accepted: January 15, 2004
Published online: November 1, 2004
AIM: To investigate the role of soluble Fas ligand in autoimmune diseases.
METHODS: RT-PCR was performed to amplify sFasL cDNA from the total RNA extracted from activated human peripheral blood lymphocytes. DNA fragments were cloned into PCR vector. After sequenced, sFasL gene fragments were inserted into pQE-31 vector and expressed in E. Coli M15 respectively. Proteins were purified through affinity chromatography column with ligand of 6 × His tag and identified by SDS-PAGE and Western blot. Mice were immunized with sFasL protein and specific anti-serum was harvested 6 wk after immunization. Monoclonal anti-human FasL antibody was made from the immunized mice. Serum level of sFasL in different patients was detected using anti-FasL antibodies from the immunized mice.
RESULTS: The protein expressed was 24 ku by SDS-PAGE electrophrosis. The protein was specially bound to anti-human FasL antibody by Western blot analysis. The sFasL protein could induce Jurket cell apoptosis in vitro. The concentration of serum sFasL in patients with autoimmune diseases was higher than that in normal individuals. sFasL could reduce arthritis in collagen induced arthritis (CIA) mice model by subcutaneous injection.
CONCLUSION: sFasL may be involved in either induction of apoptosis or autoimmune diseases. Furthermore, sFasL may have potential application in treatment of autoimmune diseases.
- Citation: Li NL, Nie H, Yu QW, Zhang JY, Ma AL, Shen BH, Wang L, Bai J, Chen XH, Zhou T, Zhang DQ. Role of soluble Fas ligand in autoimmune diseases. World J Gastroenterol 2004; 10(21): 3151-3156
- URL: https://www.wjgnet.com/1007-9327/full/v10/i21/3151.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i21.3151
Autoimmune diseases are a kind of diseases induced by auto-reactive T or B cells. Most common autoimmune diseases include rheumatoid arthritis (RA), insulin-dependent diabetes mellitus (IDDM), multiple sclerosis (MS) and systemic lupus erythematosus (SLE). Among these diseases, RA, IDDM and MS are believed to be autoimmune diseases induced by Th1 cells which can recognize auto-antigens on special tissues. For example, RA is a common disease characterized by the chronic lesion of polyarthritides. Autoimmunity to cartilage antigens may play a significant role in the pathogenesis of chronic inflammatory polyarthritis. It has been commonly accepted that cell mediated immune responses are involved in chronic inflammation since T and B lymphocytes and antigen presenting cells are observed to be enriched in the synovium fluid of RA patients. In vivo studies showed that T cells infiltrating into the synovium could express IL-2 receptors, IL-10, IFN-γ and activated CD4 T cells could be detected in the synovial fluid of RA patients[1,2]. It is believed that if auto-reactive T cells can be cleaned from circulation or RA can be located, the lesion induced by auto-reactive T cells may relieve. Actually, anti-TNF-α and anti-CD4 monoclonal antibodies have been used in treatment of RA patients.
In this study, we reported that sFasL proteins were expressed in E. Coli system. Anti-FasL antibodies were prepared in the mice using expressed FasL. Meanwhile, we set up a special ELISA method to identify sFasL molecules and its content in the serum of patients with RA, IDDM, MS and SLE. We also tried to use sFasL made in our lab oratory to treat collagen induced arthritis in this study.
Fresh blood samples were supplied by healthy individuals. PHA was purchased from Sigma Com. (San Diego, USA) IPTG and plasmid mini-prep Kit were products of Promega (Chicago, USA). DNA purification kit, Histidine resin, sequencing vector PCR2.1 and protein expression vector pQE-31 were purchased from QIAGEN (San Diego, USA). Rabbit anti-human FasL polyclonal antibody was purchased from Santa Cruz (Swedeen). Goat anti-rabbit IgG/HRP was purchased from Huamei (Shanghai, China). Nitofible membrane was purchased from Amersham (London, England). Trizol, dNTP, restricted enzymes were purchased from Gibco BRL (San Diego, USA) and MBI Fermentans (Philadophia, USA).
Peripheral blood monocytes (PBMC) were isolated from blood from healthy individuals with Ficoll. PBMC were re-suspended in 100 mL/L fetal calf serum RPMI 1640 and incubated at 37 °C in bumidified atomosphere containing 5 mL/L CO2 for 24 h. PHA (20 μg/mL) was added into the culture and incubated for another 8 h.
sFasL expression on the cell surface was detected by direct influoroscense labeling with cytometry. Briefly, activated lymphocytes were collected and washed with PBS. FITC-labeled goat anti-human FasL (Oncogen Company, LA, USA) was added into the cells and incubated on ice for 30 min. The labeled cells were washed with PBS and expression of FasL on the cell surface was detected by FACS. The percentage of expression of FasL was analyzed using system software II.
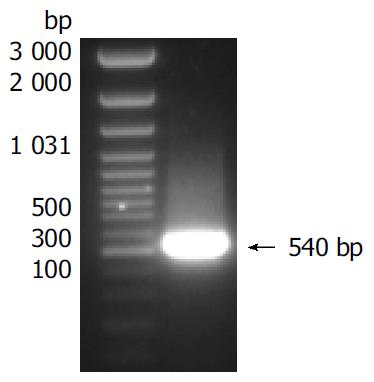
Total RNA was isolated from cells in which sFasL expression was enhanced and reversed into cDNA. sFasL gene fragments were amplified from cDNA template by special primers. sFasL: The sequence of forward primer was: 5’-ACG GAT CCG CAG CAG CCT TCA ATT A CC-3’; reverse primer: 5’-AAG CCG AAT ATA TTC GAG ATT AAG CTT CGC CG-3’. The size of sFasL fragments amplified from cDNA was 540 bp.
PCR bands were cut and purified with gel extraction kit (QIAGEN). The purified PCR products were inserted into PCR 2.1 vector and DH 5α (E. Coli) was transfected with TA cloning kit (Invitrogen) according to the manufacturers introductions. White clones were picked up on selected LB plates containing ampcillin and X-gel and expensed in 3 mL of LB medium overnight. Plasmids were prepared from the culture with mini-prep Kit (Promega). The positive clones were identified by EcoR I digested and sequenced by Shanghai South Gene Tech Ltd. The identified sFasL fragments were digested by BamHII and Hind III and purified by gel extraction kit. The purified sFasL fragments were ligated into the expression vector pQE-31 and M15 (E. Coli) was transfected. White clones were picked up on selected LB plates containing Kanamycin and expensed in 3 mL of LB medium overnight and inoculated in 200 mL of selected LB medium containing Kanamycin until A reached to 0.7. A 1 mol/L of IPTG was added to culture and shaken for another 4 h. Bacteria were collected by spinning at 6 000 r/min for 10 min and lyzed in lysis buffer. Protein expressed was purified by His Tag resin.
sFasL expressed was identified by SDS-PAGE electrophrosis and Western blot. sFasL showed a single band with Mr 24 000 respectively after running from SDS-PAGE (120 g/L concentrated gel and 40 g/L separated gel). The sFasL bands were transferred onto membranes and blocked with 50 mL/L of free fat milk for 2 h at room temperature. The membranes were washed 3 times with PBS. The rabbit anti-human sFasL polyclonal antibody was added and incubated for 1 h at room temperature. Goat anti-rabbit IgG-HRP was added and incubated for another 1 h at room temperature after washed with PBS. DAB and H2O2 were added and the reaction was stopped by washing the membranes with water when brown color was visible.
sFasL (25, 50 and 100 µg/L) was added into 1 × 10 8/L of Jurket cell lines respectively and incubated for 8 h at 37 °C in humidified atomosphere containing 5 mL/L CO2. The cells were collected and fixed by 700 mL/L ethanol for 45 min at 4 °C. Ten µL of RNase was added to the fixed cells after washed with PBS and incubated for 30 min at 37 °C. Then 10 µL of PI (50 mg/mL) was added to the cells and detected by cytometry. The percentage of apoptotic cells was calculated by apoptotic peak (A0) area.
Two Balb/C mice were immunized with 80 µg of expressed sFasL mixed with complete Frund’s adjuvent by injection with s.c on back. The mice were injected three times with sFasL, once every two weeks. Then blood was collected and anti-FasL titer was measured with ELISA.
Wistar rats were bred under pathogen-free conditions . Female mice were used at 2-3 mo of age and allowed free access to standard laboratory diet and water. Collagen II (CII) emulsion was prepared in an equel volume of IFA. A 100 µL (containing 300 µg of CII) of CII/IFA emulsion was intradermally injected, using the loose skin at the base of the tail. Seven days later, the primary immunization was administered, a booster intradermal injection containing 100 µg of CII in IFA. The rats were observed every day from the 4th wk after primary immunization. Arthritis was evaluated with standard 4 scores.
When arthritis occurred in rats immunized with CII/IFA, 1 µg of sFasL expressed in 100 µL of PBS was injected into one ankylosis side. Meanwhile 100 µL of PBS was injected into another ankylosis side of same arthritis rats as control. The injection was given three times, once every two days.
Serum level of soluble FasL in different patients was detected with indirect ELISA. The ELISA plate was coated with 100 µL of serum and incubated overnight at 4 °C. The coated plate was completely washed with PBS and prepared mouse anti-human FasL antibody was added. Rabbit anti-mouse IgG-HRP was then added and incubated for another 1 h at room temperature after washed with PBS. DAB and H2O2 were added and the reaction was stopped by washing the membranes with water when brown color was visible.
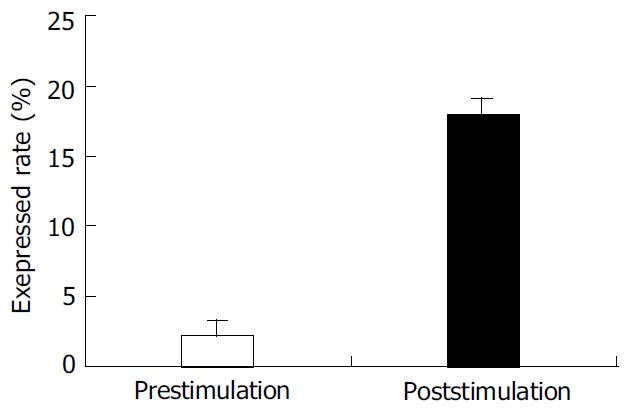
PBMC was isolated from healthy individuals FasL was expressed on the cell surface after activated with PHA. The expression of FasL in PBMC was significantly higher after activated. The percentage of FasL detected on the non-activated PBMC was very low (2.15 ± 1.7%), while FasL expressed on the activated PBMC reached 17.98 ± 1.8% using cytometry detection after cells were labeled with specific fluorescence anti-human FasL antibody (Figure 1).
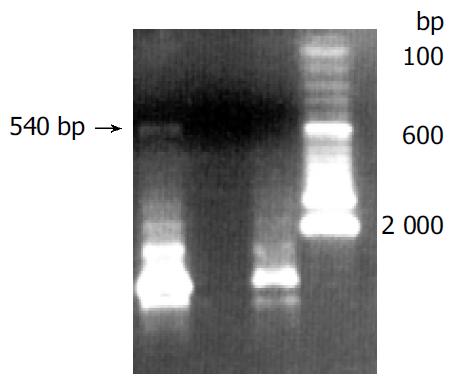
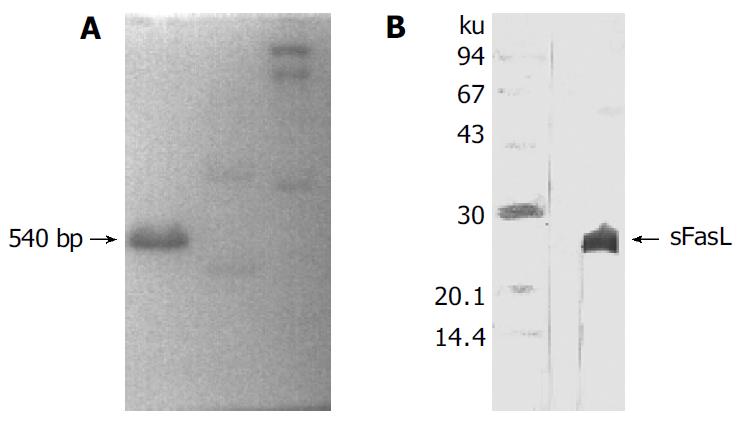
Human sFasL DNA fragments were amplified from activated PBMC using RT-PCR. The size of PCR product was 540 bp (Figure 2) which matched the size reported in the GenBank. The product was inserted into PCR2.1 vector and DH5α was successfully transfected. The insert was confirmed by digestion with EcoR I after plasmid was miniprepared. The fragments were separated with a 3.9 kb vector. This result showed that sFasL (Figure 3) fragments were successfully inserted into the vector. The sequences of the inserted fragments were same as reported by GenBank after sequenced with 377 Sequencer. Identified fragments were digested by both BamHI and Hind III and ligated into the expression vector pQE-31. The ligated products were put on selected LB plates after M15 was transfected and incubated for 18 h at 37 °C. The expression was induced by IPTG for 4 h. The protein was purified by 6-histidine column. The molecular weight and purification of the protein were identified by SDS-PAGE. The result indicated that there was only one band in sFasL (24 000 dolton). Special mouse anti-human FasL antibody was used to confirm the bands transferred onto membranes with Western blotting. The result showed there was a sFasL positive band. This indicated that the protein expressed in M15 was human sFasL (Figure 4).
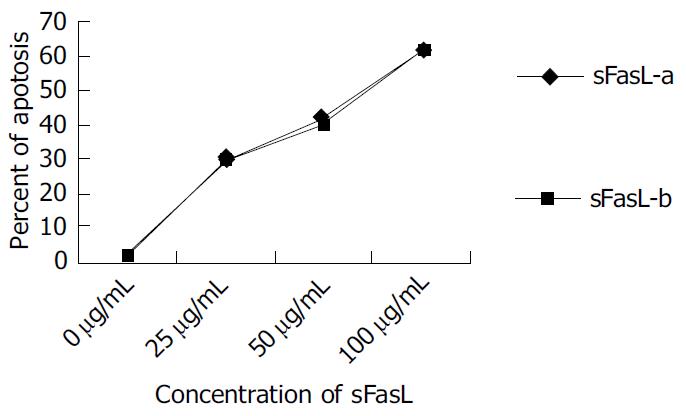
Different doses of expressed sFasL were added to log phase growing Jurkat cell line and cultured for another 18 h. The percentage of apoptosis of tested cell line was determined by cytometry after stained with PI dye. Figure 5 shows the apoptosis of Jurkat cells after co-cultured with expressed sFasL. The percentage of apoptotic Jurkat cells was similar to that of apoptotic cells co-incubated with commercial human sFasL. This result indicated that sFasL was able to induce cell apoptosis (Figure 5).
Serum was isolated from two mice immunized wirh expressed human sFasL. We coated plates with this serum and detected anti-human FasL titer using special commercial standard sFasL protein by ELISA. The result showed that there was a high titer (> 1:1 000) of anti-human sFasL special antibody in the serum of immunized mice. This indicated that expressed sFasL could specifically bind to standard human sFasL protein.
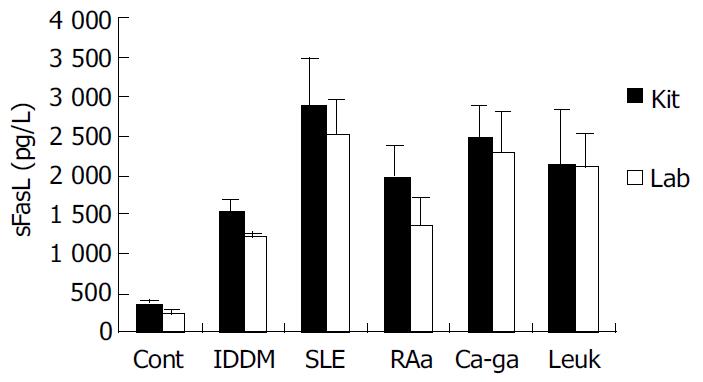
We coated ELISA plates with special anti-human sFasL antibody generated from immunized mice to detect the concentration of sFasL in the serum of patients with tumor and autoimmune diseases. Twenty serum samples from healthy individuals, 95 serum samples from patients with different autoimmune diseases and 30 serum samples from patients with leukemia and cancer were detected by this way. As a parallel experiment, the concentration of sFasL in these serum samples was identified in duplicate using commercial Kit at the same time. The data showed that the concentration of sFasL was significantly higher than that in healthy individuals (P < 0.05). The result determined with prepared sFasL antibody in our lab oratory was same as that detected by commercial Kit (Figure 6).
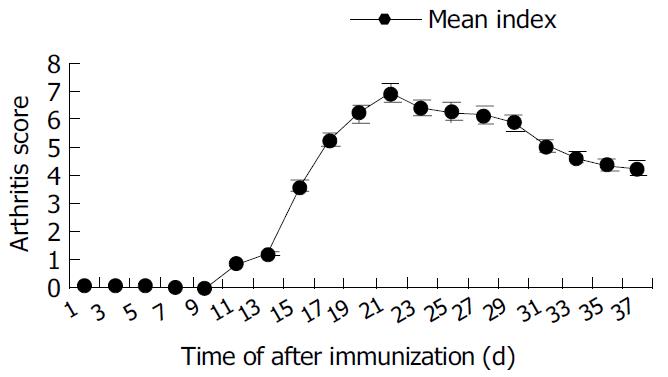
Arthritis occurred as early as 15 d after primary immunization with CII emulsified with IFA. The significant ankylosis was observed in rats. The joints of rats were red and swollen. The arthritis rats lost ability to move and showed difficult to reach food. The percentage of arthritis was 90% (Table 1). The mean score could reach the maximum 24 d after immunization began to decrease from d 30 (Figure 7).
| Group | Immunized with | Incidence | Percentage of CIA (%) |
| Experimental | CII + CFA | 18/20 | 90 |
| Control | CII | 0/5 | 0 |
| CFA | 0/5 | 0 |
Wistar rats were intradermally injected with collagen II (CII) emulsion in an equel volume of IFA 100 μL (containing 300 μg of CII). Seven days later, a booster containing 100 μg of CII in IFA intradermally administered. The rats were observed every day from 4 wk and evaluated with standard 4 scores.
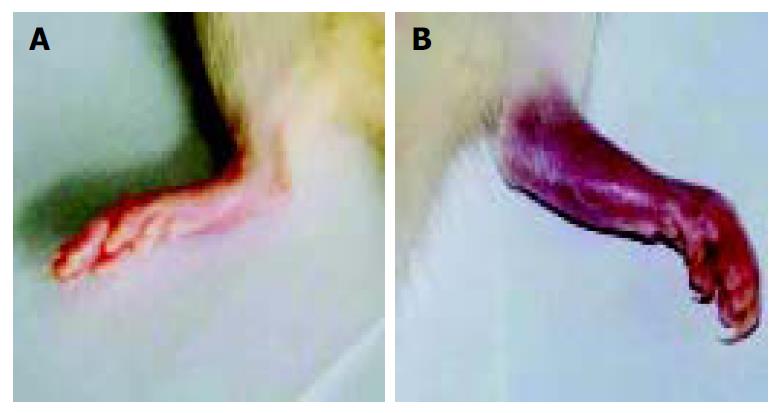
When arthritis had a score of 7-8, 1 μg of sFasL in 100 μL of PBS was injected into one ankylosis side of rats. Meanwhile, 100 μL of PBS was injected into another ankylosis side of the same arthritis rats as control. After the arthritis rats were injected 3 times with sFasL, the rats showed reducible arthritis (Figure 8A) while control rats which received an equal volume PBS suffered from a more serious ankylosis (Figure 8B).
Human Fas ligand (CD95 ligand, FasL) was identified by Nagata’s laboratory in 1993. Human FasL is consistented of 281 amino acids and a member of TNF family. The N terminal of FasL was located in the cytoplasm and C terminal is on cell surface. The region of the molecules in cytoplasm is consisted of 80 amino acids. The region though the cell membrane of FasL is consisted of 22 amino acids. The region of FasL exposed to outside of the cells is consisted of 179 amino acids[3]. Fas ligand molecules can be cut by matrix metalloproteinases (MMP) from membranes[15] and become a soluble form which is called soluble FasL (sFasL). FasL could induce apoptosis of cells when it binds to Fas which expresses on cell surface and acts as a special receptor of FasL.
Fas is ubiquitously expressed on a number of different cells including tumor cells. Likewise, as a ligand of Fas, FasL has many important functions. The expression of FasL is not only restricted to activated T cells and NK cells, but also on immune privileged sites such as eyes and testis. FasL can be up-regulated when cells are responsive to UV irradiation or treated by cytotoxic drugs. Over expression of FasL can inhibit some autoimmue diseases by deleting auto-immune memory cells[4,5]. Moreover, Fas-FasL interaction can protect allografts from immune attack and induce anti-tumor responses. Thus, myoblasts engineered to express FasL have been found to prolong the survival of co-transplanted islet allografts in diabetic mice. FasL expression on allogeneic fibroblasts was found to abolish tumor growth and induce specific protective immunity when mixed with neoplastic cells before implantation in vivo. Apoptosis induced by Fas-FasL interaction is believed to involve inflammation, anti-tumor and autoimmune reaction and aging[6-8]. Apoptosis has been found in almost all processes of the life. For example, activation induced cell death (AICD) may play a very important role in T cell activation and differentiation.
As the out-membrane region of FasL, sFasL involves the deletion of potentially hazardous peripheral memory cells[5]. Some studies suggested that change of sFasL in serum mignt be involved in many processes and progress of diseases. The serum level of sFasL was high in patients with large granule lymphocyte leukemia and NK like lymphoma, SLE, RA and EAE[9-11]. sFasL was upregulated on the tumor surface upon drug incubation in chemosensitive tumor cells. Tumor cells could mediate autocrine or paracrine to release soluble FasL (sFasL) and induce apoptosis of peripheral normal cells, CTL and NK which could express Fas and promote local tumor invasion or metastases[11-13]. Matsuno et al[14] reported that both sFas and sFasL were higher in the synovium fluid of RA patients compared with other arthritis. Serve RA showed a higher level of sFasL than mild RA[13,21]. The concentration of Fas, receptors of FasL and sFasL, were changed in the process of diseases. Some investigators reported that sFas might block apoptosis by binding FasL or sFasL[5,6]. Membrane-bound FasL induced apoptosis in cultured synovial cells from RA and OA patients, but naturally processed human sFasL did not. The role of sFasL still remains unclear.
T cells express both Fas and FasL on cell surface after stimulation. FasL existes in two forms: Membrane-bound FasL and soluble FasL (sFasL). The out-membrane region of FasL is cleaved by a metalloproteinase (MP) and falls down from membrane to serum or body fluid[14]. Inhibition of metalloproteinase cleavage could enhance the cytotoxicity of FasL[15]. Activated T cells would die in a few minutes if no IL-2 was added after stimulation with antigens or mitogens. This process is called activated induced cell death (AICD). Because sFasL shows the same function of FasL but has smaller molecules. We expressed sFasL first in E. Coli. For preparing sFasL cDNA and stimulated T cells with PHA in this study. The expression of sFasL reached 20% after stimulation for 24 h comparied with 3% expression on naïve T cells. We harvested sFasL expressed T cells before they died and mRNA of sFasL was extracted. The fresh mRNA of sFasL was reversed into cDNA and successfully amplified. The sFasL was ligated into PCR2.1 vector. After the sFasL insert was confirmed by sequencing analysis, the fragment of sFasL was inserted into pQE-31 vector and expressed in M15 strain.
To identify whether sFasL expressed in this study had biological functions after purification, we chose Jucket cell line as a report cell because Fas molecules were expressed on Jucket cell line surface. When Fas expressed on cell surface bound to sFasL, the cells were activated and went to die after 48 h of binding. The results demonstrated that the expressed sFasL could induce Jucket cell line apoptosis. The percent of apotosis increased with the increased concentration in the culture. This result indicated that sFasL had the same function membrane FasL.
Recently, some reports demonstrated that FasL and sFasL played a different role in apoptosis. Generally, membrane-bound FasL, or FasL is fully competent in transducing Fas-mediated signals for apoptosis and NF-kappaB nuclear translocation because membrane FasL could possess both Fas-focusing and signal transducing functions. While sFasL is known to be deficient in transducing signals upon engagement with membrane Fas[4,14]. But in some cases, sFasL was found to be involved in the deletion of potentially hazardous peripheral memory cells[10]. A more study suggested that change of sFasL in serum was involved in the process and progress of diseases[9]. The report indicated there was a high serum level of sFasL in patients with large granular lymphocyte leukemia and NK-like lymphoma[12]. sFasL increased significantly in the serum of patients with activated systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). In some autoimmune diseases the serum level of sFasL was dramatically decreased after treatment in some patients[10,13]. We detected the serum level of sFasL in more than 90 samples from autoimmune disease in this study. Among these diseases, the serum levels were detected from 32 patients with IDDM, 31 patients with SLE, 16 RA patients in activative stage and 16 RA patients in resting stage. This result indicated that the circulation level of sFasL was higher in these autoimmune diseases. The role of sFasL in the pathogenesis of these autoimmune diseases is still under investigation.
It has been commonly accepted that activated CD4 T cell mediated immune responses are involved in the pathogenesis of RA. Both CD4 and CD8 T cells were observed to be enriched in the synovial fluid of RA patients. It is possible that if auto-reactive T cells can be cleaned from circulation or RA can be located, the lesion caused by inflammation induced the auto-reactive T cells may relieve. Similar with RA, it is believed that Th1 is involved in the pathogenesis of CIA. Collagen induced arthritis (CIA) has become a commonl animal model in studing the pathogenesis of rheumatoid arthritis[2,17]. Many potential factors involved in CIA[2,18-20], Th1 cells are mainly involved in the CIA. If Th1 cells are deleted from joints, the evolvement of joints in CIA may be lighter. Mattsson et al[20] reported that the T cell marker O × 40 was up-regulated in OVA-inhibited non-arthritic animals compared to control CIA animals. They found that the complete inhibition of CIA caused by addition of OVA to the collagen II inoculum was due to the presence of a TH2 environment resulting from an increased production of IL-4 mRNA and a parallel increase in O × 40+ T cells [20]. In this report, we used sFasL to treat CIA mice. The anklynosis was reduced after 3 times of sFasL injection in arthritis rats. This result indicated that sFasL might induce apoptosis of activated autoimmune Th1 cells which infiltrated in joints and synovium so that the inflammation induced by autoimmune T cells decreased, or the above process increased the Th2 switch. But how sFasL induces these changes is still under investigation.
In this study, human sFas ligand was engineered in E. Coli. The expressed sFasL could induce apoptosis of Jurkat cell line. Anti-human sFasL antibody could be generated in the mice immunized with expressed sFasL. Anti-human sFasL antibody could be used in detection of sFasL in the serum of patients with autoimmune diseases. The results demonstrated that the level of sFasL in sera of patients with IDDM, SLE and RA was much higher than that in healthy individuals. Meanwhile, the result showed that sFasL antibody revealed the same sensitivity as commercial sFasL antibody. Injection of sFasL with sc in CIA rats could reduce inflammation, which may indicate that sFasL can be used in treatment of autoimmune diseases.
We thank professors Kang-Yan Zhou and Shan-Qing Tong for their critical review of this paper and Dr. Zuo-Qing Li for his assistance in preparation of this manuscript.
| 1. | Li NL, Zhang DQ, Zhou KY, Cartman A, Leroux JY, Poole AR, Zhang YP. Isolation and characteristics of autoreactive T cells specific to aggrecan G1 domain from rheumatoid arthritis patients. Cell Res. 2000;10:39-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Nandakumar KS, Andrén M, Martinsson P, Bajtner E, Hellström S, Holmdahl R, Kleinau S. Induction of arthritis by single monoclonal IgG anti-collagen type II antibodies and enhancement of arthritis in mice lacking inhibitory FcgammaRIIB. Eur J Immunol. 2003;33:2269-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 107] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Takahashi T, Tanaka M, Inazawa J, Abe T, Suda T, Nagata S. Human Fas ligand: gene structure, chromosomal location and species specificity. Int Immunol. 1994;6:1567-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 326] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 4. | Ishimaru N, Yanagi K, Ogawa K, Suda T, Saito I, Hayashi Y. Possible role of organ-specific autoantigen for Fas ligand-mediated activation-induced cell death in murine Sjögren's syndrome. J Immunol. 2001;167:6031-6037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Kim S, Kim KA, Hwang DY, Lee TH, Kayagaki N, Yagita H, Lee MS. Inhibition of autoimmune diabetes by Fas ligand: the paradox is solved. J Immunol. 2000;164:2931-2936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Hoffmann TK, Dworacki G, Tsukihiro T, Meidenbauer N, Gooding W, Johnson JT, Whiteside TL. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin Cancer Res. 2002;8:2553-2562. [PubMed] |
| 7. | Suzuki N, Ichino M, Mihara S, Kaneko S, Sakane T. Inhibition of Fas/Fas ligand-mediated apoptotic cell death of lymphocytes in vitro by circulating anti-Fas ligand autoantibodies in patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:344-353. [PubMed] [DOI] [Full Text] |
| 8. | French LE, Hahne M, Viard I, Radlgruber G, Zanone R, Becker K, Müller C, Tschopp J. Fas and Fas ligand in embryos and adult mice: ligand expression in several immune-privileged tissues and coexpression in adult tissues characterized by apoptotic cell turnover. J Cell Biol. 1996;133:335-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 343] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 9. | Zhu B, Luo L, Chen Y, Paty DW, Cynader MS. Intrathecal Fas ligand infusion strengthens immunoprivilege of central nervous system and suppresses experimental autoimmune encephalomyelitis. J Immunol. 2002;169:1561-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Christensson M, Pettersson E, Eneslätt K, Christensson B, Bratt J, Rantapää-Dahlqvist S, Sundqvist KG. Serum sFAS levels are elevated in ANCA-positive vasculitis compared with other autoimmune diseases. J Clin Immunol. 2002;22:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Kayagaki N, Kawasaki A, Ebata T, Ohmoto H, Ikeda S, Inoue S, Yoshino K, Okumura K, Yagita H. Metalloproteinase-mediated release of human Fas ligand. J Exp Med. 1995;182:1777-1783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 641] [Cited by in RCA: 640] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 12. | Nagao M, Nakajima Y, Hisanaga M, Kayagaki N, Kanehiro H, Aomatsu Y, Ko S, Yagita H, Yamada T, Okumura K. The alteration of Fas receptor and ligand system in hepatocellular carcinomas: how do hepatoma cells escape from the host immune surveillance in vivo. Hepatology. 1999;30:413-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Nozawa K, Kayagaki N, Tokano Y, Yagita H, Okumura K, Hasimoto H. Soluble Fas (APO-1, CD95) and soluble Fas ligand in rheumatic diseases. Arthritis Rheum. 1997;40:1126-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 89] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Matsuno H, Yudoh K, Watanabe Y, Nakazawa F, Aono H, Kimura T. Stromelysin-1 (MMP-3) in synovial fluid of patients with rheumatoid arthritis has potential to cleave membrane bound Fas ligand. J Rheumatol. 2001;28:22-28. [PubMed] |
| 15. | Knox PG, Milner AE, Green NK, Eliopoulos AG, Young LS. Inhibition of metalloproteinase cleavage enhances the cytotoxicity of Fas ligand. J Immunol. 2003;170:677-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Xiao S, Jodo S, Sung SS, Marshak-Rothstein A, Ju ST. A novel signaling mechanism for soluble CD95 ligand. Synergy with anti-CD95 monoclonal antibodies for apoptosis and NF-kappaB nuclear translocation. J Biol Chem. 2002;277:50907-50913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Vossenaar ER, Nijenhuis S, Helsen MM, van der Heijden A, Senshu T, van den Berg WB, van Venrooij WJ, Joosten LA. Citrullination of synovial proteins in murine models of rheumatoid arthritis. Arthritis Rheum. 2003;48:2489-2500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 185] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Banda NK, Kraus DM, Muggli M, Bendele A, Holers VM, Arend WP. Prevention of collagen-induced arthritis in mice transgenic for the complement inhibitor complement receptor 1-related gene/protein y. J Immunol. 2003;171:2109-2115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Morgan ME, Sutmuller RP, Witteveen HJ, van Duivenvoorde LM, Zanelli E, Melief CJ, Snijders A, Offringa R, de Vries RR, Toes RE. CD25+ cell depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 2003;48:1452-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 248] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 20. | Mattsson L, Lundberg K, Mussener E, Jansson A, Erlandsson Harris H, Larsson P. Antigen inhibition of collagen-induced arthritis is associated with up-regulation of IL-4 mRNA and induction of Ox40 on T cells in draining lymph nodes. Clin Exp Immunol. 2003;131:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Hashimoto H, Tanaka M, Suda T, Tomita T, Hayashida K, Takeuchi E, Kaneko M, Takano H, Nagata S, Ochi T. Soluble Fas ligand in the joints of patients with rheumatoid arthritis and osteoarthritis. Arthritis Rheum. 1998;41:657-662. [PubMed] [DOI] [Full Text] |
Edited by Wang XL and Zhang JZ Proofread by Xu FM