Published online Jan 15, 2004. doi: 10.3748/wjg.v10.i2.244
Revised: May 27, 2003
Accepted: June 4, 2003
Published online: January 15, 2004
AIM: To develop a hepatocyte targeting pH-sensitive liposome for drug delivery based on active targeting technology mediated by asialoglycoprotein receptors.
METHODS: Four types of targeting molecules with galactose residue were synthesized and mixed with pH-sensitive lipids DC-chol/DOPE to prepare liposome with integrated property of hepatocyte specificity and pH sensitivity. Liposome 18-gal was selected with the best transfection activity through cellular uptake experiment. Property analysis was made through experiments of competitive inhibition of receptors, red blood cell hemolysis, in vitro cytotoxicity test by MTS assay and mediation of inhibitory effects of antisense phosphorothioate ODN on gene expression, etc.
RESULTS: Liposome 18-gal had the desired properties of hepatocyte specificity, pH sensitivity, low cytotoxicity, and high transfection efficiency.
CONCLUSION: Liposome 18-gal can be further developed as a potential hepatocyte- targeting delivery system.
- Citation: Wen SY, Wang XH, Lin L, Guan W, Wang SQ. Preparation and property analysis of a hepatocyte targeting pH-sensitive liposome. World J Gastroenterol 2004; 10(2): 244-249
- URL: https://www.wjgnet.com/1007-9327/full/v10/i2/244.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i2.244
Previous studies have suggested a promising future of antisense oligodeoxynucleotide (ODN) in the treatment of viral hepatitis[1-6]. However, as a polyanionic macromolecule, antisense ODN has two major limitations: poor efficiency of cellular uptake and rapid degradation. The realization of its in vivo biological effect is accomplished under the mediation of liposome. In recent years, with the advent of cationic liposome and active targeting technology[7-10], liposome technology has been widely applied in the transfer of antisense ODNs for its virtues of high transfection efficiency, protection for the entrapped and ease of chemical modification.
The effectiveness of cationic liposome in the mediation of antisense ODN transfer in clinical trials was reported[11-15], while its deficiency lay in the lack of tissue specificity. To improve the targeting property, active targeting technology based on specific receptor-ligand recognition reaction has been applied. Hepatocytes exclusively expressed large numbers of high affinity asialoglycoprotein receptors which can recognize the ligand molecules with galactose residues and mediate their endocytosis[16-20]. In this study, four types of targeting molecules bearing galactose residue were synthesized and mixed with the pH-sensitive lipids DC-chol/DOPE to prepare liposome with integrated property of hepatocyte specificity and pH sensitivity.
Chloroformylcholesterol, N,N-dimethylethylenediamine, dioleoylphosphatidylethanol- amine, hydrolytic lactose were purchased from Sigma Chemicals (St. Louis, MO), DC-chol was synthesized according to the published methods[21]. Enzymes, vecters, luciferase assay system and Celltiter 96®AQueous non-radioactive cell proliferation assay test kit were obtained from Promega Corp (Madison, WI). All other reagents were analytically pure.
Plasmid and transgeneic cell line HepG2.9706[22] pHCV-neo4 was constructed by cloning the complete 5’NCR (non-coding region) and part C region of fusion protein of Chinese HCV genome into pGL3 luciferase reporter vector in which the start codon was deleted without frameshift. In pHCV-neo4, the expression of luciferase gene could be suppressed by blocking the HCV 5’ NCR. Transgenic cell strain HepG2. 9706 with permanent expression of luciferase gene was constructed by selection of the transfected HepG2 cell with pHCV-neo4 using G418.
Phosphorothioate ODN HCV363a (5’ GAG-GTT-TAG-GAT-TCG-TGC-TCA-TG 3’) is a 23-mer sequence complimentary to part of HCV 5’ NCR (noncoding region). HCV363s (5’ CAT-GAG-CAC-GAA-TCC-TAA-ACC-TC 3’) is the reverse complimentary sequence to HCV363a. NSC (non-specific control: 5’ GCA-GAG-GTG-AAA-AAG-TTG-CAT 3’) is a random sequence with low homology to HCV5’NCR. The oligonucleotides were synthesized with the automatic DNA synthesizer (390Z, Applied Biosystems, Inc) and purified by HPLC (Micro Pure II reverse phase column).
Four types of amphiphilic glycolipid molecules (octodecyl galactoside, octodecyl lactoside, cholesteryl galactoside, cholesteryl lactoside) bearing galactose residues were synthesized according to the published method[23,24] with some modifications. Hydrophilic groups were galactose and lactose, and hydrophobic groups were octadecanol and cholesterol. Structure confirmation was made through element analysis, nuclear magnetic resonance and mass spectrum (data not shown).
Targeting molecule: DC-chol: DOPE (optimum:6:4 molar ratio) was dissolved in the solvent mixture of ethanol/chloroform (1:1 v/v), evaporated to a thin film in a rotating evaporator, resuspended in the sterile PBS buffer (pH7.4) and incubated overnight at 4 °C with gentle stirring. The suspension was sonicated in a bath sonicator for 30 minutes and passed through a polycarbonate membrane (pore diameter 0.4 μm) filter for sterility and granular uniformity. The particle size of the liposomes was measured in a dynamic light scattering spectrophotometer (LS-900, Otsuka Electronics, Osaka, Japan). Lipid concentration in stock solution was determined by phosphorus assay.
Cells (HepG2, GLC) were maintained in DMEM supplement with 10% FBS at 37 °C under an atmosphere of 5% CO2 in air. The cells were seeded onto a 96-well plate at a density of 104/well and cultivated in 0.2 mL DMEM supplemented with 10% FBS. After 24 h, the culture medium was replaced with DMEM containing plasmid DNA/liposome complexes. Five hours later, the incubation medium was replaced again with DMEM supplemented with 10% FBS and incubated for an additional period of 36 h. The cells were then collected and washed with PBS buffer twice, mixed with 30 μL/well cell lysis solution. Ten minutes later, 100 μL/well luciferase substrate buffer was added and the light produced was immediately measured using a luminometer. The activity was indicated as the relative light units per mg protein. The protein content of the cell suspension was determined by a modified Lowry method using BSA as a standard. In the experiment of competitive inhibiton of receptors, the galactose/glucose solution was added 5 minutes before plasmid DNA/liposome complexes, and all other procedures were the same. All assays were performed in triplicate.
A series of 0.1 mol/L PBS buffer with gradient pH value (4.0, 5.0, 6.0, 7.0, 8.0) were prepared. Eighty μL 1%(v/v) newly prepared chicken red blood cell suspension and 20 μL plasmid DNA/liposome complexes (1:5 wt/wt, lipid concentration: 0.5 μg/μL) were mixed in 100 μL 0.1M PBS buffer of different pH value. The mixture was shaken at 37 °C, and an aliquot of suspension was taken at the given time periods (10 min, 30 min and 90 min), centrifuged at 1 000×g. Absorbance value of the supernatant was measured at wavelength 540nm in a photometer (Multiscan MS). Controls were set as follows:
Parallel control: 100 μL PBS buffer + 80 μL1%(v/v) RBC + 20 μL saline;
Negative control: 120 μL saline + 80 μL1% (v/v)RBC.
DNA/liposome (1:5 wt/wt) complexes were diluted with DMEM supplemented with 2% FBS in an index gradient way (index number: 2, initial lipid concentration: 0.5 μg/μL). HepG2 cells were seeded onto a 96-well plate at a density of 104/well and cultivated in 0.2 mL DMEM supplemented with 10% FBS. After 24 h, the culture medium was replaced with DMEM containing DNA/liposome complexes prepared, and cells were incubated for an additional period of 24 h. Cytotoxicity was evaluated by MTS assay according to the instructions of Celltiter 96®AQueous non-radioactive cell proliferation assay test kit, and commercially available cationic liposome Lipofectin served as the control.
Phosphorothioate ODNs were dissolved in DMEM at various concentrations (0.2 μmol/L, 0.4 μmol/L and 0.8 μmol/L), alone or in combination with liposome18-gal (octadecanol-galactoside:DC-chol:DOPE 1:6:4) at a ratio of 1:5 (wt/wt). The HepG2.9706 cells seeded onto a 24-well plate at a density of 2×104/well were incubated in DMEM supplement with 10% FBS. After 24 h, the culture medium was replaced with DMEM containing phosphorothioate ODNs. Five hours later, the incubation medium was replaced again with DMEM supplemented with 10% FBS and incubated for an additional period of 24 h. Then the activity of luciferase enzyme was determined as in the plasmid transfection experiment.
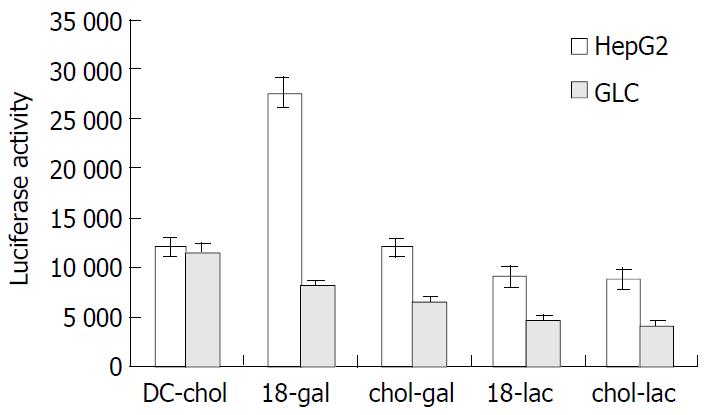
Four types of targeting liposomes (denoted as 18-gal, 18-lac, chol-gal and chol-lac respectively) were prepared by mixing glycolipids with DC-chol/DOPE in a molar ratio of 1:6:4, respectively. A fixed amount of (0.4 μg/well) plasmid DNA encoding a luciferase gene was complexed with these liposomes at a ratio of 1:5 (wt/wt). Figure 1 shows the expression of lucifease gene in HepG2 cells (liver cells) and GLC cells (lung cells lacking asialoglycoprotein receptor) treated with the DNA/liposome complexes. The transfection activity of DC-chol/DOPE was similar in the two cell lines, whereas the transfection activity of the targeting liposomes was significantly higher in HepG2 cells than in GLC cells. When compared with the transfection activity of DC-chol/ DOPE in HepG2 cells, there was a significant increase in that of 18-gal (P < 0.01) while a relatively significant decrease in those of 18-lac and chol-lac (P < 0.05), and no significant difference was observed between those of chol-gal and DC-chol/DOPE (P > 0.05). In GLC cells, the transfection activity of the targeting liposomes was significantly lower than that of DC-chol/DOPE (P < 0.05). These results suggested that the addition of pooly soluble and electrically neutral glycoside molecules could impaire the transfection activity of DC-chol/ DOPE to some extent. However, asialoglycoprotein receptor-mediated internalization induced by targeting molecules with galactose residues could specifically enhance the transfection activity of targeting liposomes in HepG2 cells, especially that of 18-gal.
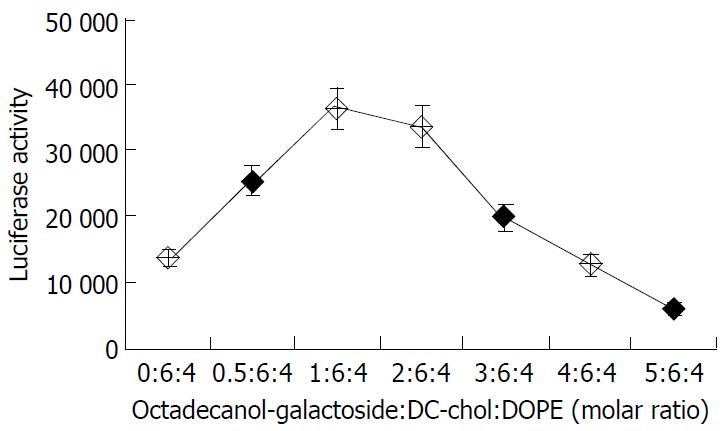
The following experiments were performed with liposome 18-gal. To optimize the lipid composition, the molar ratio of octadecanol-galactoside to lipid DC-chol/DOPE varied from 0:6:4 to 5:6:4. The liposomal transfection activity showed a bell-shape dependence on the molar percentage of octadecanol-galactoside in liposomal composition. The maximum gene expression (about 3 times that of DC-chol/DOPE) was observed at the ratio of octadecanol-galactoside:DC-chol:DOPE 1:6:4 (Figure 2). When the molar percentage of octadecanol-galactoside exceeded 20%, the colloidal solution was easy to agglomerate into turbidity, and the liposomal tansfection efficiency dropped significantly. Therefore, the maximum molar proportion of octadecanol-galactoside in the liposomal formula should not exceed 20 mol%.
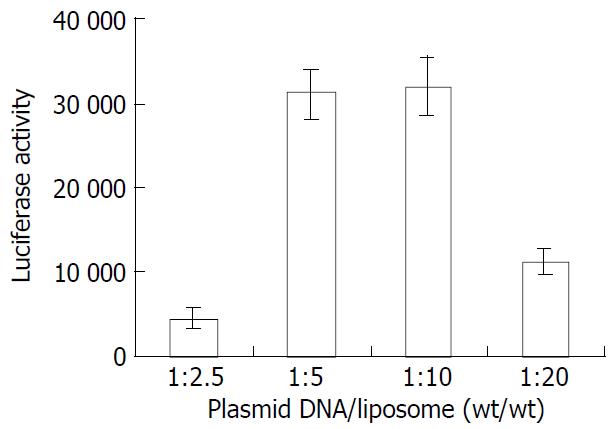
A fixed amount of plasmid DNA (0.4 μg/well) was complexed with liposome 18-gal (octadecanol-galactoside:DC-chol:DOPE 1:6:4) in different ratios (1:2.5, 1:5, 1:10, 1:20 wt/wt) to treat HepG2 cells. The greatest gene expression was achieved at the ratio of 1:5-1:10 (Figure 3).
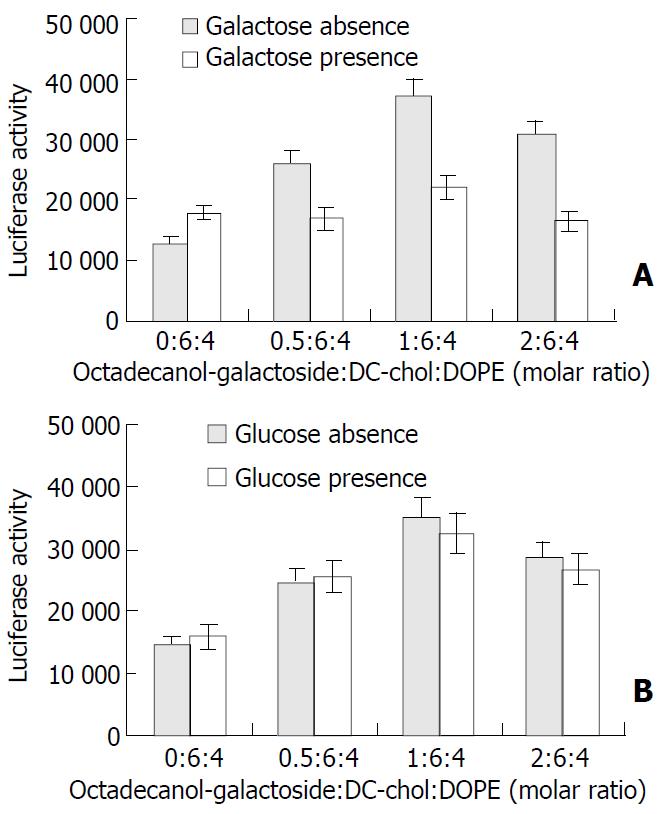
To investigate whether the cellular uptake of liposome 18-gal in HepG2 cells was partly mediated by asialoglycoprotein receptors, the inhibitory effect of 20 mmol/L galactose solution on the transfection activity of liposome 18-gal of different compositions (the ratio of octadecanol-galactoside to DC-chol/ DOPE ranging from 0:6:4 to 2:6:4) was measured. As shown in Figure 4A, the transfection efficiency of liposome 18-gal was significantly inhibited (P < 0.01, the mean inhibition rates were 35%, 40% and 46% respectively) in the presence of galactose, but not that of DC-chol/DOPE(6:4). On the other hand, no significant difference was found in the gene expression of DNA/liposome 18-gal complexes in the presence or absence of glucose (Figure 4B).
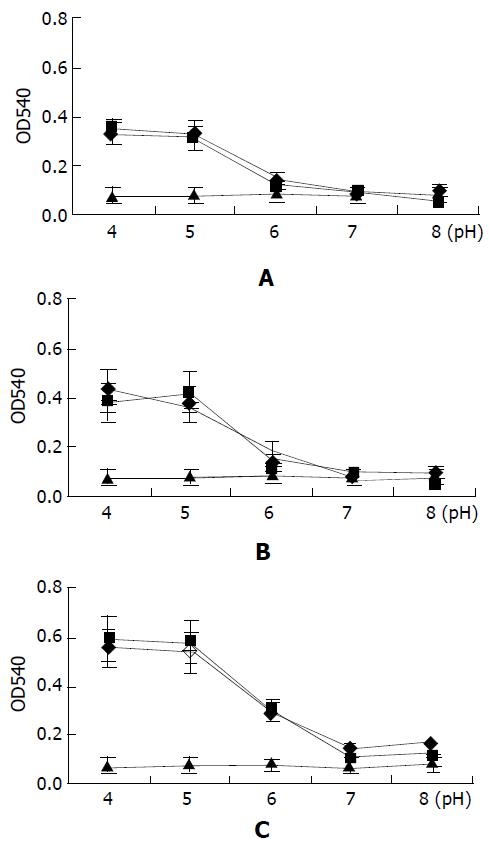
To characterize the liposomal pH sensitivity, plasmid DNA/ liposome complexes were mixed with chicken hematocyte in different pH conditions, and release of hemachrome which indicates cell membrane fusion was determined at 10 min, 30 min and 90 min. As shown in Figure 5, there was no difference (P > 0.05) at the release of hemachrome in the PBS buffer of various pH values. They were close to the negative control (the mean value of negative control: 10 min, 0.076; 30 min, 0.077; 90 min, 0.082). The membrane fusion of red blood cells with DNA/liposome complexes was significantly dependent on the pH value. There was a significant difference between the release amounts of hemachrome in the intervals before and after pH = 6 (P < 0.01).
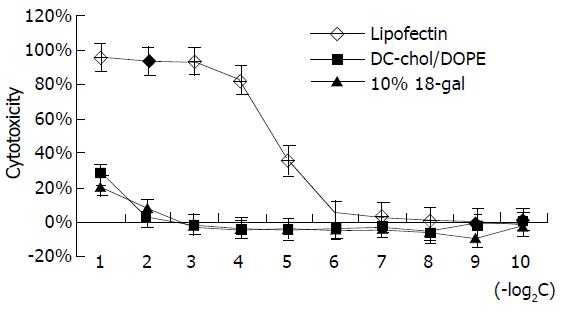
The cytotoxicity of liposome 18-gal was tested and compared with that of lipofectin. As shown in Figure 6, within lipid concentrations of 0.5, 0.25, 0.125 and 0.0625 μg/μL, the cytotoxicity of lipofectin was significantly higher than that of liposome 18-gal and DC-Chol/DOPE at corresponding concentrations, the latter two only demonstrated certain cytotoxicity at the concentration of 0.5 μg/μL (about 25%). When the concentration that generated 25% cytotoxicity (IC25) was compared, the cytotoxicity of lipofectin was 16 (24) times higher than that of the other two liposomes. This results confirmed that DC-Chol/DOPE was a type of cationic liposome with low cytotoxicity, and the addition of octadecanol-galactoside in the liposomal formula did not increase the cytotoxicity.
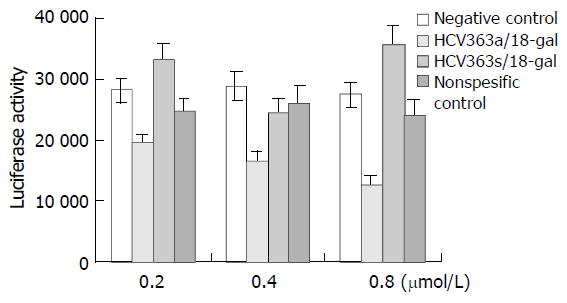
HepG2.9706 cells were treated with phosphorothioate ODNs at different concentrations (0.2, 0.4 and 0.8 μmol/L), alone or in combination with liposome 18-gal. As shown in Figure 7, within the range of 0.2-0.8 μmol/L, HCV363a/18-gal complexes had a significantly dose-dependent inhibitory activity on the expression of luciferase gene, and the inhibition rate was 31%, 43% and 54% respectively. HCV363s/18-gal complexes showed stimulating effects on gene expression to some extent. The stimulating effects of sense oligonucleotides on gene expression were also reported by other researchers[25,26], but the cause has been unclear. NSC/18-gal complexes had nonspecific inhibitory effects on gene expresson of no more than 15%. Treatment with phosphorothioate ODNs alone showed no inhibitory effects on the expression of luciferase gene in HepG2.9706 within the concentration of 0.8 μmol/L.
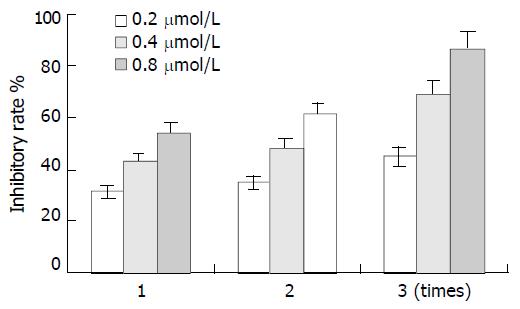
In addition, the effect of administration time on the inhibitory activity of HCV363a/18-gal complexes was also investigated. HepG2.9706 cells were consecutively treated 3 times with HCV363a/18-gal complexes and the activity of luciferase enzyme was determined each time 24h after HCV363a/18-gal complexes were administered. As shown in Figure 8, the inhibitory effects of HCV363a/18-gal complexes increased with the administration time.
In the present study, we synthesized targeting molecules with galactose residue and developed a novel formula for a hepatocyte-targeting pH sensitive liposome. Liposome 18-gal was selected with the best transfection activity that was greatly mediated by asialoglycoprotein receptor in HepG2 cells. The hepatocyte specificity of liposome 18-gal was confirmed by the following facts: (1) There was a significant difference (P < 0.01) in the transfection efficiencies of DNA/18-gal complexes between HepG2 cells (liver cells) and GLC cells (lung cells lacking asialoglycoprotein receptors). (2) Within a certain proportion (< 20 mol%), the addition of octadecanol-galactoside greatly increased the transfection efficiency of DC-chol/DOPE in HepG2 cells (maximum: about 3 folds). This effect was significantly inhibited by galactose but not by glucose. In comparison, the transfection activity of DC-chol/ DOPE was similar in the presence or absence of galactose or glucose in HepG2 cells.
In the presence of excessive proportion of targeting molecules (> 20 mol%, molar percentage in liposome composition), the colloidal solution was easy to form turbidity, and the transfection efficiency of prepared liposomes dropped significantly. It was because that a large amount of electrically neutral targeting molecules reduced the positive charge density of cationic liposome. Studies with phospholipid containing liposomes have shown that an increase in a glycolipid or gangliosides greater than 10 mol% resulted in the solubility of liposome into micelles. Due to the potential shielding of the cationic lipid interaction with plasmid DNA by the carbohydrate head group, a heterogeneous population of mixed micelles could be generated at a high molar percent, thus explaining the bell shaped curve for expression. The poor solubility of glycoside molecules in organic solvent also affected the preparation and property of liposome to some extent.
pH-sensitivity is another important property of liposome, which was considered as a mechanism to amass the enclosed antisense ODN at the action site of cytoplasm[27-29]. In brief, liposome is selectively uptaken by specific cells based on active targeting mechanism. The pH sensitivity inducing the liposomal escape from endosome/lysosome at low pH value makes the entrapped antisense ODN release at cytoplasm to take action. In the present study, the significant dependence on the pH value of membrane fusion of chicken hematocytes with DNA/liposome complexes confirmed the pH sensitivity of the two liposomes, and the similarity of the two liposomes in the memberane fusion experiment strongly indicated the pH-sensitivity of liposome 18-gal as that of DC-chol/DOPE which has been proved to be a pH sensitive liposome[30].
Transgenic cell strain HepG2.9706 with permanent expression of luciferase gene is a convenient cell model purposely constructed to evaluate the inhibitory effect of antisense ODNs on the HCV 5’ NCR. The comparison between the effects on gene expression of phosphorothiote ODNs with or without liposome 18-gal mediation showed that liposome 18-gal was highly efficient in mediating the delivery of phosphorothioate ODNs into HepG2.9706 cells.
In conclusion, the liposome 18-gal prepared in this study has the desired properties of hepatocyte specificity, pH sensitivity, low cytotoxicity, and high transfection efficiency. It can be further developed as a potential hepatocyte-targeting ODN delivery system.
| 1. | Heintges T, Encke J, zu Putlitz J, Wands JR. Inhibition of hepatitis C virus NS3 function by antisense oligodeoxynucleotides and protease inhibitor. J Med Virol. 2001;65:671-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Robaczewska M, Guerret S, Remy JS, Chemin I, Offensperger WB, Chevallier M, Behr JP, Podhajska AJ, Blum HE, Trepo C. Inhibition of hepadnaviral replication by polyethylenimine-based intravenous delivery of antisense phosphodiester oligodeoxynucleotides to the liver. Gene Ther. 2001;8:874-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Alt M, Eisenhardt S, Serwe M, Renz R, Engels JW, Caselmann WH. Comparative inhibitory potential of differently modified antisense oligodeoxynucleotides on hepatitis C virus translation. Eur J Clin Invest. 1999;29:868-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Alt M, Renz R, Hofschneider PH, Caselmann WH. Core specific antisense phosphorothioate oligodeoxynucleotides as potent and specific inhibitors of hepatitis C viral translation. Arch Virol. 1997;142:589-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Alt M, Renz R, Hofschneider PH, Paumgartner G, Caselmann WH. Specific inhibition of hepatitis C viral gene expression by antisense phosphorothioate oligodeoxynucleotides. Hepatology. 1995;22:707-717. [PubMed] |
| 6. | Liu Y, Chen Z, He N. [Inhibition of hepatitis C virus by antisense oligodeoxynucleotide in vitro]. Zhonghua Yi Xue Za Zhi. 1997;77:567-570. [PubMed] |
| 7. | Zabner J. Cationic lipids used in gene transfer. Adv Drug Deliv Rev. 1997;27:17-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 108] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Kim JK, Choi SH, Kim CO, Park JS, Ahn WS, Kim CK. Enhancement of polyethylene glycol (PEG)-modified cationic liposome-mediated gene deliveries: effects on serum stability and transfection efficiency. J Pharm Pharmacol. 2003;55:453-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Abra RM, Bankert RB, Chen F, Egilmez NK, Huang K, Saville R, Slater JL, Sugano M, Yokota SJ. The next generation of liposome delivery systems: recent experience with tumor-targeted, sterically-stabilized immunoliposomes and active-loading gradients. J Liposome Res. 2002;12:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Mastrobattista E, Koning GA, Storm G. Immunoliposomes for the targeted delivery of antitumor drugs. Adv Drug Deliv Rev. 1999;40:103-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 118] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Hyde SC, Southern KW, Gileadi U, Fitzjohn EM, Mofford KA, Waddell BE, Gooi HC, Goddard CA, Hannavy K, Smyth SE. Repeat administration of DNA/liposomes to the nasal epithelium of patients with cystic fibrosis. Gene Ther. 2000;7:1156-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 159] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Stopeck AT, Jones A, Hersh EM, Thompson JA, Finucane DM, Gutheil JC, Gonzalez R. Phase II study of direct intralesional gene transfer of allovectin-7, an HLA-B7/beta2-microglobulin DNA-liposome complex, in patients with metastatic melanoma. Clin Cancer Res. 2001;7:2285-2291. [PubMed] |
| 13. | Hortobagyi GN, Ueno NT, Xia W, Zhang S, Wolf JK, Putnam JB, Weiden PL, Willey JS, Carey M, Branham DL. Cationic liposome-mediated E1A gene transfer to human breast and ovarian cancer cells and its biologic effects: a phase I clinical trial. J Clin Oncol. 2001;19:3422-3433. [PubMed] |
| 14. | Hui KM, Ang PT, Huang L, Tay SK. Phase I study of immunotherapy of cutaneous metastases of human carcinoma using allogeneic and xenogeneic MHC DNA-liposome complexes. Gene Ther. 1997;4:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Gill DR, Southern KW, Mofford KA, Seddon T, Huang L, Sorgi F, Thomson A, MacVinish LJ, Ratcliff R, Bilton D. A placebo-controlled study of liposome-mediated gene transfer to the nasal epithelium of patients with cystic fibrosis. Gene Ther. 1997;4:199-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 162] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Gao S, Chen J, Xu X, Ding Z, Yang YH, Hua Z, Zhang J. Galactosylated low molecular weight chitosan as DNA carrier for hepatocyte-targeting. Int J Pharm. 2003;255:57-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 109] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Kunath K, von Harpe A, Fischer D, Kissel T. Galactose-PEI-DNA complexes for targeted gene delivery: degree of substitution affects complex size and transfection efficiency. J Control Release. 2003;88:159-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 112] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Yuan L, He S, Guan C, Pang Q. [The preparation and study on hepatic targeting tendency of galactosyl-anti-CD3-McAb in mice]. Hua Xi Yi Ke Da Xue Xue Bao. 2001;32:424-426. [PubMed] |
| 19. | Kawakami S, Yamashita F, Nishikawa M, Takakura Y, Hashida M. Asialoglycoprotein receptor-mediated gene transfer using novel galactosylated cationic liposomes. Biochem Biophys Res Commun. 1998;252:78-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 119] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Hara T, Kuwasawa H, Aramaki Y, Takada S, Koike K, Ishidate K, Kato H, Tsuchiya S. Effects of fusogenic and DNA-binding amphiphilic compounds on the receptor-mediated gene transfer into hepatic cells by asialofetuin-labeled liposomes. Biochim Biophys Acta. 1996;1278:51-58. [RCA] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Gao X, Huang L. A novel cationic liposome reagent for efficient transfection of mammalian cells. Biochem Biophys Res Commun. 1991;179:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 639] [Article Influence: 18.3] [Reference Citation Analysis (6)] |
| 22. | Wang XH, Wang SQ, Li MD, Zhu BZ. Establishment of a HCV 5' NCR transgenic cell model. Bingdu Xuebao. 1998;14:296-301. |
| 23. | Smits E, Ernberts FN, Kellogg RM. Reliable method for the syn-thesis of aryl b-D-glucopyranosides, using boron trifluoride-di-ethyl ether as catalysts. J Chem Soc Perkin Trans. 1996;2873-2877. |
| 24. | Lubineu A, Queneau Y. Aqueous cycloadditions using gluco-organic substrates 1. stereo- chemical course of the reaction. J Org Chem. 1987;52:1001-1009. [RCA] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Wakita T, Wands JR. Specific inhibition of hepatitis C virus expression by antisense oligodeoxynucleotides. In vitro model for selection of target sequence. J Biol Chem. 1994;269:14205-14210. [PubMed] |
| 26. | Chen J, Simon RP, Nagayama T, Zhu R, Loeffert JE, Watkins SC, Graham SH. Suppression of endogenous bcl-2 expression by antisense treatment exacerbates ischemic neuronal death. J Cereb Blood Flow Metab. 2000;20:1033-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Venugopalan P, Jain S, Sankar S, Singh P, Rawat A, Vyas SP. pH-sensitive liposomes: mechanism of triggered release to drug and gene delivery prospects. Pharmazie. 2002;57:659-671. [PubMed] |
| 28. | Simões S, Slepushkin V, Düzgünes N, Pedroso de Lima MC. On the mechanisms of internalization and intracellular delivery mediated by pH-sensitive liposomes. Biochim Biophys Acta. 2001;1515:23-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 113] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Simões S, Slepushkin V, Pires P, Gaspar R, de Lima MP, Düzgüneş N. Mechanisms of gene transfer mediated by lipoplexes associated with targeting ligands or pH-sensitive peptides. Gene Ther. 1999;6:1798-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 120] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Wrobel I, Collins D. Fusion of cationic liposomes with mammalian cells occurs after endocytosis. Biochim Biophys Acta. 1995;1235:296-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 253] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
Edited by Ma JY and Wang XL