INTRODUCTION
Osteopontin (OPN) is a secreted arginine-glycine-aspartate (RGD)-containing phosphoprotein with cell adhesive and chemotactic properties in vitro and in vivo[1-3]. It is closely associated with infiltrating macrophages in tumors and can directly stimulate macrophage migration, which has made it a key target as a molecule likely to be important in mediating tumor metastasis[4]. It has been shown that osteopontin is up-regulated in many kinds of cancer, including hepatocellular carcinoma[5,6], breast cancer[7-9], prostate cancer[10,11], ovarian cancer[12,13], brain cancer[14,15] and lung cancer[16]. Elevated osteopontin transcription often correlates with increased metastatic potential of cancers.
Epidermal growth factor (EGF) receptor (EGFR) is a member of the ErbB family of ligand-activated tyrosine kinase receptors, which play a central role in the proliferation, differentiation, and/or oncogenesis of epithelial cells, neural cells, and fibroblasts[17,18]. It has been reported that EGF can induce osteopontin expression of breast cancer cells[19], rat kidney epithelial cells[20] and HL60 cells[21], and the induction of osteopontin may involve in signaling pathway related to PKC and tyrosine kinase[22]. The mechanism responsible for osteopontin up-regulation in HCC is unknown, but may involve induction by specific cytokines. We have investigated this hypothesis by testing the effects of epidermal growth factor on osteopontin regulation in hepatocellular carcinoma cell line, HepG2. Using RNase protection assay, Western blot, we found that HepG2 cells constitutively expressed low levels of osteopontin mRNA and protein. EGF is a potent inducer of osteopontin mRNA and protein in HepG2 cells. Wortmannin, a specific PI3-K inhibitor, blocks the EGF-induced OPN expression. Our study suggests that OPN expression induced by EGF is dependent on PI3-K signaling pathway in HepG2 cells.
MATERIALS AND METHODS
Cell line and culture
HepG2 cell line was obtained from Cell Biology Institute (Shanghai, China). Cells were cultured in DMEM (Gibcol BRL) containing 10% fetal calf serum (Hyclone).
Induction of growth factor signaling
Cells were plated at 1×105 per well in 6-well plates, and were grown overnight. Next morning, the cultures were washed twice with PBS and maintained in serum-free medium (containing 0.05% BSA) for 24 hours. The cells were then stimulated with the indicated amounts of EGF (Sigma) for the indicated time frames before harvesting and analysis.
To assess signal transduction molecules involved in the induction of osteopontin gene expression, the PI3-Kinase inhibitor wortmannin (Calbiochem) was added to the cells. The cells were pretreated with respective inhibitors or vehicle (DMSO) alone for half an hour and then treated in combination with EGF for 8 hours. Preliminary dose-response experiments had defined the concentrations of 100nM wortmannin to be effective and non-toxic.
DNA constructs and in vitro transcription
Plasmid pGEM-OPN containing osteopontin fragments was constructed in our laboratory. A 486bp of OPN fragment was obtained by reverse transcription-PCR from plasmid pBlueScript-OPN containing full length human osteopontin (a kind gift from Dr. Chambers, Canada) using the sense primer 5’-ATGGATCCGATGACACTGATGATTCTCAC-3’and antisense primer 5’-GCGAATTCGAATTCACGGCTGACAAA-3’. The resultant BamH1-EcoR1 cDNA fragment was ligated into the vector pGEM, and confirmed by direct sequencing.
To make RNA probes for OPN and β-actin (a linearized plasmid containing β-actin fragment included in the kit), in vitro transcription was performed with a commercial kit (Ambion), according to the user’s instructions.
RNA isolation and RNase protection assay
Total RNA was isolated using the RNeasy Mini kit (QIAGEN GmbH, Germany). RNase protection assay was performed with a commercial kit (Ambion). α32P-UTP labeled probes were mixed with sample RNA and co-precipitated. Hybridization was proceeded for 10 minutes at 68 °C, followed by digestion with RNase A-T1 for 30 minutes at 37 °C, and separation of hybridized RNA on 5% acrylamide and 8M urea gels. The gels were dried on filter paper for 40 minutes at 80 °C, and exposed to X-ray film. The autoradiographs were scanned using an AlphaImager 2200 spot densitometer (Alpha Innotech Corporation), and the integrated densities of areas were recorded.
Western blot
Cultured cells were lysed in RIPA buffer (50 mM Tris-HCL PH7.5, 150 mM NaCl, 1% NP-40, 0.5% Na-deoxycholate, 0.1% sodium dodecyl sulfate). Cell lysates were denatured at 100 °C for 5 minutes, and equal amounts of protein were loaded onto 10% SDS-polyacrylamide gels. The separated proteins were transferred to PVDF membranes (ROCHE) and probed with antibodies to osteopontin (Calbiochem). Membranes were stripped and re-probed with antibodies to tubulin as an internal control.
RESULTS
OPN expression in HepG2 cells induced by EGF-treatment
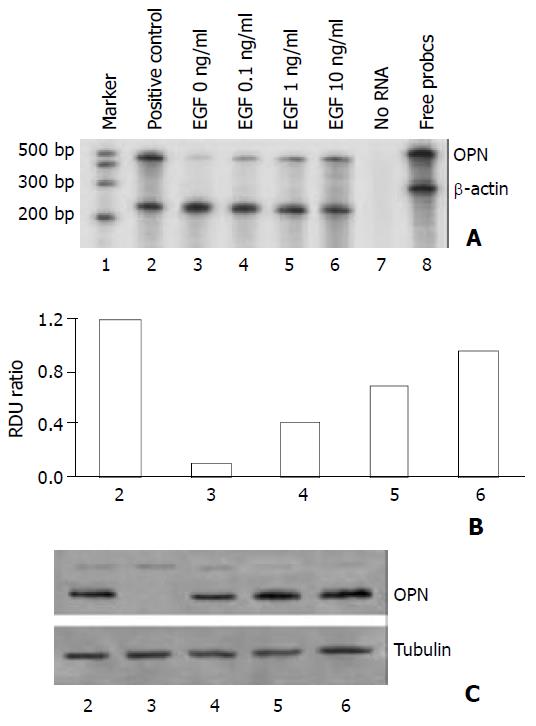
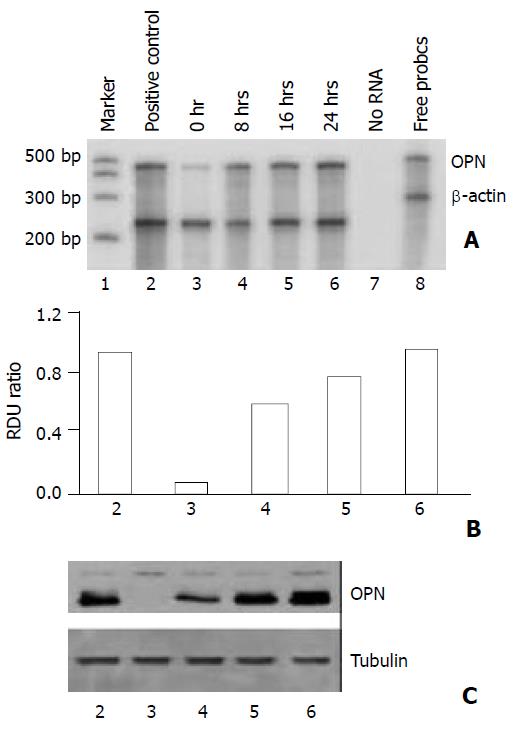
In order to examine the regulators of osteopontin expression in hepatocellular carcinoma cells, HepG2 cells were incubated in serum-free medium and then treated with epidermal growth factor that had been implicated in HCC. As shown in Figures 1 and 2, HepG2 cells constitutively expressed low levels of osteopontin, stimulation of the cells with EGF increased osteopontin mRNA expression as well as protein level in a dose-dependent and time-dependent manner. To quantitate this finding, densitometry of osteopontin mRNA levels was recorded (Figure 1B and Figure 2B). Osteopontin expression was elevated after treatment with EGF within a 8-hour period, expression levels were further increased at 16 hours and 24 hours following treatment.
Figure 1 Dose-dependent effect of EGF on osteopontin expres-sion in HepG2 cells.
1×105 cells were plated in 6-well plates. Next morning, cell cultures (near 80% confluent) were incu-bated in serum-free medium for 24 hours. The cells were stimu-lated dose-dependently by epidermal growth factor. Positive control denotes cells in normal growth medium containing 10% fetal calf serum. A. Osteopontin mRNA level was analyzed by RNase protection assay on total RNA using a 486 base-pair probe and controlling the loading with a probe for β-actin. B. Quantitation of osteopontin mRNA levels is shown in (A). RDU ratio reflects relative density units of osteopontin mRNA di-vided by β-actin mRNA. C. The results from RNase protection assay were confirmed by Western blot of osteopontin protein. Tubulin was served as loading control.
Figure 2 Time course showing effect of EGF on osteopontin expression in HepG2 cells.
1×105 cells were plated in 6-well plates. Next morning, cell cultures (near 80% confluent) were incubated in serum-free medium for 24 hours. The cells were stimulated time-dependently by 10 ng/ml EGF. Positive con-trol denotes cells in normal growth medium containing 10% fetal calf serum. A. Osteopontin gene expression was analyzed by RNase protection assay on total RNA using a 486 base pair probe and standardized by comparison to β-actin. B. Quantitation of osteopontin mRNA levels is shown in (A). RDU ratio reflects relative density units of osteopontin mRNA di-vided by β-actin mRNA. C. Western blot of osteopontin pro-tein by cell lysates confirmed the results from RNase protec-tion assay. Tubulin was served as loading control.
Interference of EGF-induced osteopontin gene expression in HepG2 cells with inhibition of PI3-kinase
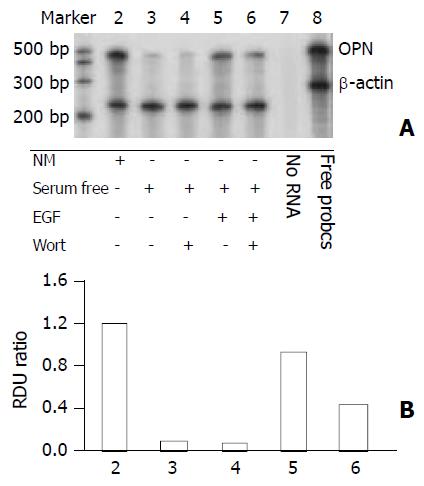
We analyzed the involvement of EGF signaling pathway in the induction of osteopontin gene expression. Addition of the PI3-kinase inhibitor wortmannin blocked EGF-mediated increase of osteopontin mRNA levels in HepG2 cells (Figure 3). It should be mentioned that wortmannin only partially reversed EGF-induced osteopontin expression, suggesting that other pathways related to EGF might involve the induction of osteopontin expression.
Figure 3 Phosphatidylinositol 3-kinase pathway dependent of osteopontin expression in HepG2 cells.
The PI3-kinase in-hibitor wortmannin was used to assess EGF-dependent sig-nal transduction leading to osteopontin gene expression. 1×105 cells were plated in 6-well plates. Next morning, cell cultures (near 80% confluent) were incubated in serum-free medium for 24 hours. 100 nM wortmannin was added to the cells 30 minutes before incubation in the presence or absence of 10 ng/ml EGF. A. Osteopontin mRNA level was analyzed by RNase protection assay on total RNA using a 486 base-pair probe and controlling the loading with a probe for β-actin. B. Quantitation of osteopontin mRNA levels is shown in (A). RDU ratio reflects relative density units of osteopontin mRNA divided by β-actin mRNA.
DISCUSSION
Hepatocellular carcinoma (HCC) is one of the most malignant tumors in the world[23]. In China, at least 100 000 new cases occur every year, and the estimated number of HCC-related deaths exceeds 110 000 per year. Recent studies indicate that the incidence of HCC in the US and UK has increased substantially over the last two decades[24,25]. Although remarkable advances in surgery have improved the prognosis of HCC patients, the high rate of intra-hepatic recurrence and metastasis remains a major challenge in HCC therapy[26,27]. Recently, it has been reported metastasis genes or metastasis-associated genes are involved in the migration and dissemination of cancers through certain signaling pathway[28,29]. Therefore, if the signal transduction pathway that regulates the expression of metastasis genes could be defined, some candidate targets via the pathway can be used to block the malignancy-promoting effects of metastasis genes, which will be useful for therapeutic intervention, including controlling the recurrence and metastasis of HCC.
Cytokine osteopontin, one of the metastatic genes, has been found in a number of tumors[5-16], and to be essential for the dissemination of various cancers[30]. Transfection of the cells with anti-sense OPN RNA reduced the malignancy of the cells and caused the decrease of tumorgenesis[31], while transfection with osteopontin increased their malignant phenotype[32]. Osteopontin was up-regulated in hepatocellular carcinoma and the increase of OPN expression was correlated with the metastatic ability of HCC and invasiveness of liver tumor–derived cell lines in vitro[5,6]. In the present study, we found that HepG2 cells constitutively expressed low levels of osteopontin, and EGF induced osteopontin expression in a dose- and time- dependent manner in the cell line. The concentration of EGF in the cells was very low. The induction of osteopontin was prominent at 0.1 ng/ml EGF concentration in our study, while the concentration of EGF was high up to 100 ng/ml in the literature[19,33]. The difference can be caused by many kinds of reasons, one of them may be that the different cells have different response to EGF. To our knowledge, this is the first study to demonstrate that EGF can cause the induction of osteopontin mRNA and protein levels in liver cancer cells. In other cell lines, the up-regulation of osteopontin by EGF was confirmed by overwhelming number of studies, including in kidney epithelial cells[33] and osteoblasts[22].
We then investigated the mechanism of osteopontin regulation by EGF in HepG2 cells. Using wortmannin, a specific inhibitor of PI3K, we found that EGF-induced osteopontin expression was significantly down-regulated. It suggested that the induction of osteopontin by EGF was dependent on PI3K signal transduction pathway in HepG2 cells. On the other hand, our results showed that wortmannin could not totally block EGF-induced osteopontin expression in HepG2 cells, suggesting that other pathways associated with EGF and its receptor may involve in the osteopontin induction.
A conclusion may be drawn from the study that EGF/PI3K signal pathway can regulate the expression of osteopontin in hepatocellular carcinoma cell line, HepG2. Several candidate targets via this pathway can be used for therapeutic intervention of HCC.