Published online Oct 1, 2004. doi: 10.3748/wjg.v10.i19.2911
Revised: October 20, 2003
Accepted: October 27, 2003
Published online: October 1, 2004
AIM: To evaluate the therapeutic effectiveness of oxaliplatin on human gastric carcinoma and to explore its mechanisms.
METHODS: Twenty-two cases of stage IV gastric carcinoma received 4-6 (mean 4.6) cycles of first line combined chemotherapy with oxaliplatin (oxaliplatin 85 mg/m2, iv, gtt, 1 h, d 1; leukovorin 200 mg/m2, iv, gtt, 1 h, d 1 and d 2; 5-FU 300 mg/m2,iv, d 1 and d 2, 5-FU, continuous iv, gtt, 48 h; 1 cycle/2 wk). Response rate, progression-free survival (PFS), total survival time, toxic side effects were evaluated. The inhibitory effect of oxaliplatin on human gastric cell line SGC-7901 was detected and IC50 was calculated by MTT. Transmission electron microscopy, flow cytometry and TUNEL were performed to evaluate the apoptosis of cell line induced by the drug. The expression of Caspase-3 m-RNA was detected by RT-PCR. AC-DEVD-CHO, a Caspase-3 specific inhibitor, was used to elucidate the role of activated Caspase-3 in the process of apoptosis induced by oxaliplatin.
RESULTS: Total response (complete and partial) occurred in 9 (40.9%) patients. Mean PFS was 4.2 mo and mean total survival time was 7.2 mo. Cumulative neurotoxicity (all grade I-II), vomiting and diarrhea, myelosuppression appeared in 93.5%, 20%, 32.9% patients, respectively. IC50 was calculated to be 0.71 mg/L by MTT assay. A maximal inhibitory rate reached 85.3%. Apoptosis index was elevated after incubated with 1 mmol/L oxaliplatin for 30 min, but without statistic significance (P > 0.05). However it could be detected at a much higher degree both by flowcytometry and by TUNEL with a statistical significance (68.47% ± 7.92% and 8.23% ± 2.67%, respectively, P < 0.05) after incubated with 1 mmol/L oxaliplatin for 2 d. By means of RT-PCR, we detected an enhancement of Caspase-3 m-RNA expression induced by oxaliplatin which was also in positive correlation with the apoptotic level. AC-DEVD-CHO, a Caspase-3 specific inhibitor, could significantly inhibit and delay apoptosis induced by oxaliplatin.
CONCLUSION: Oxaliplatin is effective and well-tolerated in patients with advanced gastric carcinoma. Oxaliplatin could significantly inhibit the growth of human gastric cell line SGC-7901. The induction of Caspase-3 m-RNA expression, activation of Caspase-3 and promotion of apoptosis may be some of the therapeutic mechanisms of oxaliplatin on gastric carcinoma. Annexin-V-fluorescein labeling flow cytometry is much more sensitive than TUNEL in detecting early stage apoptosis.
- Citation: Lin WL, Li DG, Chen Q, Lu HM. Clinical and experimental study of oxaliplatin in treating human gastric carcinoma. World J Gastroenterol 2004; 10(19): 2911-2915
- URL: https://www.wjgnet.com/1007-9327/full/v10/i19/2911.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i19.2911
Gastric cancer is one of the common carcinomas in human being. Drug treatment draws more and more attention as an essential part of comprehensive treatment of gastric malignancy. Gastric carcinoma is relatively sensitive to chemotherapy. It is generally considered that chemotherapy may prolong patient’s life and decrease relapse. Oxaliplatin (L-OHP) is an innovative third generation platinum compound with powerful anti-neoplasm competence, lack of cross drug resistance with CDDP, with a synergistic effect with 5-FU and satisfactory safety profile. This new anticancer drug provides us more choices in fighting against malignancy, especially colon cancer. At present, treating gastric cancer with oxaliplatin and the relationship between chemotherapy and cancer cell apoptosis draw more and more attention. The discovery of Caspase family (cysteine proteases) that is implicated in the execution of programmed cell death in organisms ranging from nematodes to humans, brings the fresh air to the research of malignant cell apoptosis. The Caspase family is big and family members interact with each other to promote or inhibit the process of apoptosis. Caspase-3 locates in the downstream of the Caspase cascade. The proteolytic activation of Caspase-3 plays a key role in apoptotic process. This article summarizes the effect and side effects of chemotherapy with oxaliplatin on 22 cases of stage IV human gastric cancer, and tries to elucidate the mechanisms of chemotherapy by detecting apoptosis of cancer cells and evaluating the role Caspase-3 plays in apoptotic process.
A total of 22 cases of stage IV human gastric cancer patients who underwent chemotherapy in the Affiliated Xinhua Hospital of Shanghai Second Medical University from January 1999 to September 2002 were enrolled in this study. There were 17 men and 5 women, and their age ranged from 25 to 70 years (mean, 60 ± 10 years). Among the 22 patients, 16 had poorly differentiated adenocarcinoma and 6 had signet ring cell carcinoma.
Each case received a combination chemotherapy containing L-OHP ( L-OHP 85 mg/m2 by continuous intravenous infusion for 2 h on d 1, leucovorin 200 mg/m2 by continuous intravenous infusion for 1 h on d 1 and d 2, 5-FU 300 mg/m2 by bolus intravenous injection on d 1 and d 2, 5-FU 1200 mg/m2 by continuous intravenous infusion for 48 h, one course lasting 2 wk for 4-6 courses).
Human gastric adenocarcinoma cell line SGC-7901, purchased from the Shanghai Institute of Cell Biology, Chinese Academy of Sciences, was routinely maintained in RPMI 1640 containing 100 mL/L fetal bovine serum (FBS), 100 U/mL penicillin, 100 U/mL streptomycin at 37 °C in a humidified atmosphere containing 50 mL/L CO2.
Cells were seeded at the density of 5 × 103 per well in 96-well plates in RPMI-1640 containing 100 mL/L FBS. After 24 h, fresh medium was added, containing oxaliplatin at concentrations of 0 to 10 mg/L. After 48 h incubation, MTT assay was performed, 150 µL of stock MTT (0.5 mg/mL) was added to each well, and the cells were further incubated at 37 °C for 4 h. The supernatant was removed and 150 µL DMSO was added to each well. An ELISA reader was used to measure the absorbance at a wavelength of 525 nm.
The cells treated with 0.1 mg/L oxaliplatin were trypsinized and harvested after 24 h. Subsequently the cells were fixed in 40 g/L glutaral and immersed with Epon 821, embedded in capsules and converged for 72 h at 60 °C, then prepared into ultrathin sections (60 nm) and stained with uranyl acetate and lead citrate. Cell morphology was examined by transmission electron microscopy.
SGC-7901 cells were treated with oxaliplatin or oxaliplatin plus AC-DEVD-CHO at oxaliplatin concentrations of 0 to 10 mg/L for 30 min. Cells were digested by 2.5 g/L trypsin, washed in 0.01 mol/L PBS, fixed by cold alcohol at 4 °C and dyed with annexin-V (according to the description of annexin-V kit), and then analyzed by flow cytometry.
SGC-7901 cells were added to 6-well plates with cover glass-slides at 6 × 104 cells/well, after incubated with oxaliplatin or oxaliplatin plus AC-DEVD-CHO at different oxaliplatin concentrations of 0 to 10 mg/L and fixed in 40 g/L formaldehydum polymerisatum for 1 h. After washed in 0.01 mol/L PBS twice, the cells were treated with reaction buffer, labeled with fluorescein dUTP in a humid box for 1 hour at 37 °C, then combined with anti-fluorescein antibody, colorized with NBT/BCIP. Cells were visualized with light microscopy. The apoptotic index (AI) was calculated as follows: AI = (number of apoptotic cells/total number) × 100%.
Total RNA was extracted from cells using an RNA extraction reagent, TR IZOL (Life Technologies, USA), according to standard acid-guanidium-phenol-chloroform method[17]. About 4 µg of total RNA as reversely transcribed at 42 °C for 60 min in a total of 30 µL reaction volume using a first-strand cDNA synthesis kit (Boehringer Mannheim, Germany). cDNA was incubated at 95 °C for 5 min to inactivate the reverse transcriptase, and served as template DNA for 28 rounds of amplification using the GeneAmp PCR system 2400 (Perkin-Elmer Applied Biosystems, CA, USA). PCR was performed in a standard 25 µL reaction mixture consisting of 1.5 mmol/L magnesium chloride (pH8.3), 2.5 mmol/L dNTPs, 12.5 pmoL each of sense and antisense primers and 2.5 U of Taq DNA polymerase (M BI, Canada). Amplification was performed for 1 min at 94 °C, for 1 min at 62 °C and for 1 min at 72 °C after heat-start for 5 min. Finally, an additional extension step was carried out for 10 min at 72 °C. As control, the DNA template of Caspase-3 was replaced by that of β-actin in the reaction. The amplification products were separated on 12 g/L agarose gels and visualized by ethidium bromide staining. PCR primers for Caspase-3 were as follows: forward primer, 5’-ATGGAGAACACTGAAAACTCA-3’; reverse primer , 5’-TTAGTGATAAAAATAGAGTTC-3’: according to the Caspase-3 gene structure in GeneBank. An 834 bp PCR product of Caspase-3 and a 315 bp product of β-actin were obtained.
The difference between each two groups was analyzed by ANOVA. P < 0.05 was considered statistically significant.
Nine cases achieved objective responses (including 1 complete response and 8 partial responses), the response rate was 40.9% progression-free survival (PFS) 4.2 mo, and total survival time 7.2 mo. The rate of accumulative neurotoxicity, vomiting and diarrhea, bone marrow depression was 93.5%, 20% and 32.9%, respectively (Table 1).
| Side effects | Anemia | Neutropenia | Thrombo-cytopenia | Nausea& vomiting | Diarrhea | Mucositis | Dysaesthesia |
| I | 27 | 17 | 7 | 14 | 16 | 9 | 89 |
| II | 7 | 6 | 3 | 6 | 3 | 1 | 6 |
| III | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| IV | 0 | 0 | 0 | 0 | 0 | 0 | - |
| Incidence (%) | 32.9 | 22.9 | 10 | 20 | 20 | 10 | 93.5 |
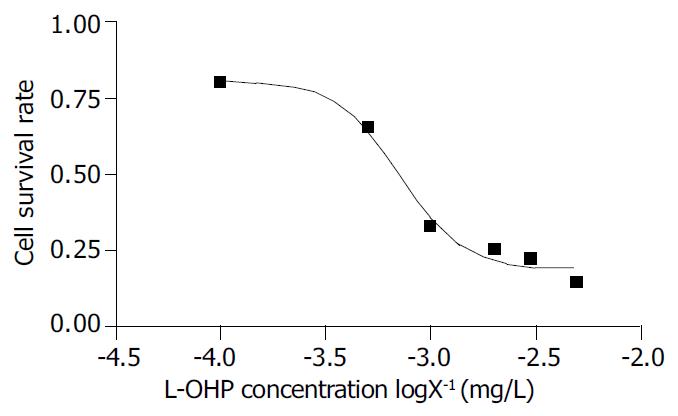
Taking the means of data from MTT assay, we got a smooth inhibition curve, which was a typical inverse ‘S’, and the IC50 was calculated to be 0.71 mg/L by GrapHpaol Prism software (Figure 1). The inhibition of L-OHP on SGC-7901 cell line was typically dose dependent. A maximal inhibitory rate reached 85.3%.
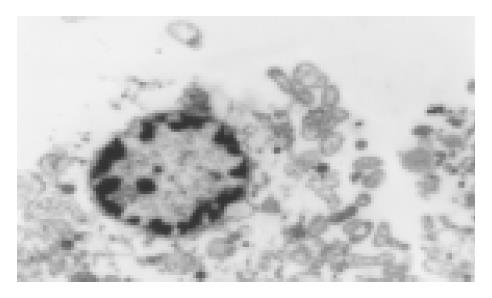
We used transmission electron microscopy, TUNEL and Annexin-V labeling flowcytometry to quest for the mechanism of its anti-neoplastic effect. After treatment of SGC-7901 cells with oxaliplatin (0.1 mg/L) for 24 h, some cells showed apoptotic characteristics including chromatin condensation, chromatin crescent formation, nucleus fragmentation and apoptotic body formation by transmission electron microscopy (Figure 2). Apoptotic index was 0.38% in the control group. In the experimental group, the apoptotic index determined by the TUNEL method was 7.35% while receiving L-OHP 1mg/L (slightly higher than IC50) for 4 h and 14.35% while increasing the L-OHP concentration to 5 mg/L. As time went on, the apoptotic index remained stable in the control group, and was significantly increased in two experimental groups (0.5 mg/L and 1 mg/L) which reached a peak of 7.93% and 10.15%, respectively on the 4th d and decreased slightly on the 7th d. The two experimental groups had a similar trend. We could conclude that the increase in apoptotic index correlated with the L-OHP concentration and time. The apoptotic level was positively correlated with L-OHP at a concentration within 0-2.0 mg/L, as detected by Annexin-V labeling flowcytometry. The apoptotic index reached a peak of 76.47% when the concentration of L-OHP was 2 mg/L. On the contrary, the apoptotic index dropped when the concentration reached 5 mg/L.
By means of RT-PCR, we detected an enhancement of Caspase-3m-RNA expression (0.48 ± 0.47 vs 0.18 ± 0.20, P < 0.05) induced by L-OHP which was also in positive correlation with the apoptotic level.
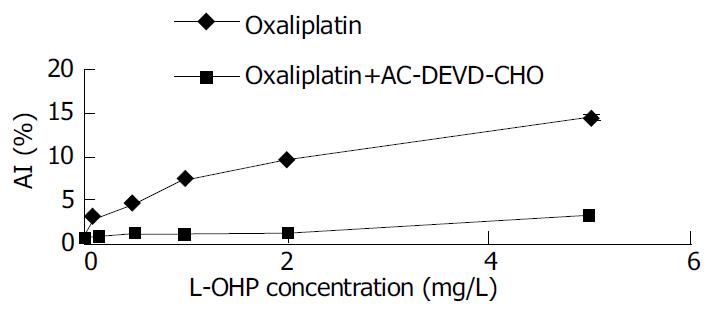
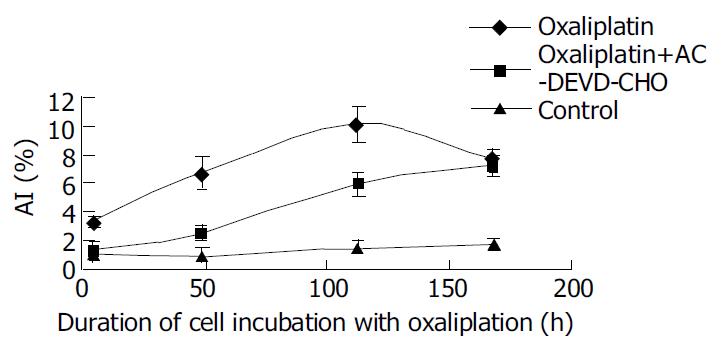
AC-DEVD-CHO, a Caspase-3 specific inhibitor, could significantly inhibit and delay apoptosis induced by L-OHP (Figure 3, Figure 4).
Gastric carcinoma is one of the major causes of cancer morbidity and mortality in China. The natural history shows a high metastatic potential since many patients with gastric carcinoma at advanced stage will relapse or initially present with metastasis. One of the first issues solved by clinical research over the last decade is the value of chemotherapy in the metastatic setting. Indeed, chemotherapy has been shown to have a favorable impact on survival and quality of life compared with supportive care alone. However, in this disease some traditional chemotherapy regimens were considered as poorly tolerated or less effective[1-4]. Oxaliplatin is an innovative platinum compound indicated as a first-line therapy in combination with 5-FU and folinic acid for metastatic colorectal cancer[5-8]. Oxaliplatin has a powerful anti-neoplasm competence, little cross drug resistance with CDDP, a synergistic effect with 5-FU and a satisfactory safety profile[9-11]. We replaced CDDP with oxaliplatin in a traditional FLP protocol, trying to explore its anti-neoplasm activity and side effects in treating advanced gastric carcinoma. In 22 patients, 9 cases achieved objective responses (including 1 complete response and 8 partial responses), the overall response rate reached 40.9%, PFS 4.2 mo, and overall survival time 7.2 mo. The toxicity was tolerable, the rate of vomiting and diarrhea (1 case with grade III diarrhea), bone marrow depression was 20% and 32.9%, respectively. No alopecia and skin toxicity were encountered. Although the incidence of accumulative neurotoxicity was as high as 93.5%, all of them were grade I-II. Acute symptoms manifesting as transient dysaesthesia and/or paraesthesia of the extremities were commonly observed, their occurrence was triggered or enhanced by exposure to cold. No patient experienced pharyngolaryngeal dysaesthesia characterized by a transient sensation of difficulty in breathing or swallowing without any objective evidence of respiratory distress, which was encountered during the multi-center research in treating colorectal cancer after 9 cycles[12-14]. In all the cases in this study, symptoms improved after treatment discontinuation. It is suggested that oxaliplatin is effective and well-tolerated in patients with stage IV gastric carcinoma.
We chose human gastric cancer cell line SGC-7901 for experimental study. First, we used MTT to prove if L-OHP could inhibit SGC-7901 growth. using the means of our data from the experiments, we obtained a smooth inhibition curve, which was a typical inversed ‘S’. IC50 was 0.71 mg/L and the maximal inhibitory rate reached 85.3%.The inhibition of L-OHP on SGC-7901 cell line was typically dose dependent.
Naturally occurring or programmed cell death can regulate cell number, facilitate morphogenesis, remove harmful or otherwise abnormal cells, and eliminate cells that have already performed their functions during the life development as well as in tissue homeostasis and aging. The role of apoptosis in the process of carcinogenesis, development of cancer and malignancy treatment has drawn more and more attention in recent years[15-17]. In this study, we tried to evaluate the level of apoptosis induced by oxaliplatin. Transmission electron microscopy could reveal the changes of cell ultrastructure during the apoptotic process. TUNEL assay is a traditional method for detecting apoptosis, but its selectivity is poor. It could hardly differentiate the apoptotic cells from the necrotic ones. Phosphatidylserine (PS) only exists in the cytoplasm side of cell plasma membrane, and externalization of PS occurs in the early stage of apoptosis. Annexin-V could specifically conjugate to the PS to detect the apoptotic cells[18]. So we combined traditional transmission electron microscopy and TUNEL assay with relatively highly selective annexin-V labeling flowcytometry to detect SGC-7901 cell line apoptosis induced by oxaliplatin. After treatment of SGC-7901 cells with oxaliplatin, some cells showed typical morphologic changes of apoptosis including chromatin condensation, chromatin crescent formation, nucleus fragmentation and apoptotic body formation under transmission electron microscope, TUNEL assay showed the AI positively correlated with drug concentration and treatment time. Annexin-V-fluorescein labeling flowcytometry was much more sensitive than TUNEL in detecting the early stage apoptosis. The apoptotic level positively correlated with L-OHP at a concentration within 0-2.0 mg/L, as detected by annexin-V labeling flowcytometry. The apoptotic index reached a peak, when the concentration of L-OHP was 2 mg/L. On the contrary, the apoptotic index dropped while the concentration reached 5 mg/L. To put these results together, we believed that the induction of apoptosis played a key role in inhibiting malignant cells at a low drug concentration of L-OHP, and that cytotoxicity and apoptosis coexisted while the drug concentration was high. This discovery may provide a theoretical basis for this type of treatment.
The discovery of cytosolic aspartate-specific proteases, called Caspases, which are responsible for the deliberate disassembly of a cell into apoptotic bodies, brings the fresh air to the research of malignant cell apoptosis. The Caspase family is big and dozens of family members interact with each other to promote or inhibit the process of apoptosis. Caspases are present as inactive pro-enzymes, most of which are activated by proteolytic cleavage. There are two pathways of Caspase activation, namely the cell surface death receptor pathway and the mitochondria-initiated pathway. In the cell surface death receptor pathway, activation of Caspase-8 following its recruitment to the death-inducing signaling complex (DISC) is the critical event that transmits the death signal. This event is regulated at several different levels by various viral and mammalian proteins. Activated Caspase-8 can activate downstream Caspases, such as Caspase-3 by direct cleavage or by indirectly cleaving bid and inducing cytochrome C release from the mitochondria. In the mitochondrial-initiated pathway, Caspase activation is triggered by the formation of an Apaf-1/cytochrome C complex that is fully functional in recruiting and activating proCaspase-9. Activated Caspase-9 will then cleave and activate downstream Caspases such as Caspase-3, -6, and -7[19-23]. So we can find out that Caspase-3 locates in the downstream of the Caspase cascade. The proteolytic activation of Caspase-3 has been found to play a key role in apoptotic process[24-26]. Caspase-3 may then cleave vital cellular proteins or activate additional Caspases by proteolytic cleavage. In this study, we detected an enhancement of Caspase-3 m-RNA expression induced by oxaliplatin by means of RT-PCR. Caspase-3 m-RNA expression was also positively correlated with the apoptotic level. AC-DEVD-CHO, a Caspase-3 specific inhibitor, could significantly inhibit and delay apoptosis induced by oxaliplatin. Taken together, we believe that Caspase-3 synthesis and activation play a key role in the apoptotic process of SGC-7901 cell line induced by oxaliplatin.
Our research demonstrates that oxaliplatin is effective and well tolerable in treating gastric cancer. Inducing cancer cell apoptosis may be one of the anti-neoplasm mechanisms. This apoptosis may be mediated by up-regulation of Caspase-3 synthesis and activation. The efficacy and safety profile of oxaliplatin as a chemotherapeutic drug in combined anti-gastric carcinoma chemotherapy regimen should be further confirmed by double blind multi-center clinical studies.
| 1. | Cascinu S, Scartozzi M, Labianca R, Catalano V, Silva RR, Barni S, Zaniboni A, D'Angelo A, Salvagni S, Martignoni G. High curative resection rate with weekly cisplatin, 5-fluorouracil, epidoxorubicin, 6S-leucovorin, glutathione, and filgastrim in patients with locally advanced, unresectable gastric cancer: a report from the Italian Group for the Study of Digestive Tract Cancer (GISCAD). Br J Cancer. 2004;90:1521-1525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Park YH, Ryoo BY, Choi SJ, Kim HT. A phase II study of capecitabine and docetaxel combination chemotherapy in patients with advanced gastric cancer. Br J Cancer. 2004;90:1329-1333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Yamamura Y, Kodera Y, Tanemura H, Oshita H, Miyashita K, Fujimura T. [A phase I study of combination chemotherapy using TS-1 and pirarubicin (THP) for advanced gastric cancer]. Gan To Kagaku Ryoho. 2004;31:361-365. [PubMed] |
| 4. | Ramos-De la Medina A, Salgado-Nesme N, Torres-Villalobos G, Medina-Franco H. Clinicopathologic characteristics of gastric cancer in a young patient population. J Gastrointest Surg. 2004;8:240-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Braun AH, Achterrath W, Wilke H, Vanhoefer U, Harstrick A, Preusser P. New systemic frontline treatment for metastatic colorectal carcinoma. Cancer. 2004;100:1558-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Santini D, Massacesi C, D'Angelillo RM, Marcucci F, Campisi C, Vincenzi B, Pilone A, Bianco V, Bonsignori M, Tonini G. Raltitrexed plus weekly oxaliplatin as first-line chemotherapy in metastatic colorectal cancer: a multicenter non-randomized phase ii study. Med Oncol. 2004;21:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Di Leo A, Buyse M, Bleiberg H. Is overall survival a realistic primary end point in advanced colorectal cancer studies? A critical assessment based on four clinical trials comparing fluorouracil plus leucovorin with the same treatment combined either with oxaliplatin or with CPT-11. Ann Oncol. 2004;15:545-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Link K, Happich K, Schirner I, Jüngert B, Brückl V, Männlein G, Brückl WM, Merkel S, Göhl J, Hohenberger W. Palliative second-line treatment with weekly high-dose 5-fluorouracil as 24-hour infusion and folinic acid (AIO) plus oxaliplatin after pre-treatment with the AIO-regimen in colorectal cancer (CRC). Anticancer Res. 2004;24:385-391. [PubMed] |
| 9. | Arnould S, Hennebelle I, Canal P, Bugat R, Guichard S. Cellular determinants of oxaliplatin sensitivity in colon cancer cell lines. Eur J Cancer. 2003;39:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 123] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Marchetti P, Galla DA, Russo FP, Ricevuto E, Flati V, Porzio G, Ficorella C, Cifone MG. Apoptosis induced by oxaliplatin in human colon cancer HCT15 cell line. Anticancer Res. 2004;24:219-226. [PubMed] |
| 11. | Ravaioli A, Marangolo M, Pasquini E, Rossi A, Amadori D, Cruciani G, Tassinari D, Oliverio G, Giovanis P, Turci D. Bolus fluorouracil and leucovorin with oxaliplatin as first-line treatment in metastatic colorectal cancer. J Clin Oncol. 2002;20:2545-2550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Chiara S, Nobile MT, Gozza A, Taveggia P, Heouaine A, Pastrone I, Percivale PL, Lionetto R, Sanguineti O, Rosso R. Phase II study of weekly oxaliplatin and high-dose infusional 5-fluorouracil plus leucovorin in pretreated patients with metastatic colorectal cancer. Anticancer Res. 2004;24:355-360. [PubMed] |
| 13. | Lehky TJ, Leonard GD, Wilson RH, Grem JL, Floeter MK. Oxaliplatin-induced neurotoxicity: acute hyperexcitability and chronic neuropathy. Muscle Nerve. 2004;29:387-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 231] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 14. | Cavaletti G, Petruccioli MG, Marmiroli P, Rigolio R, Galbiati S, Zoia C, Ferrarese C, Tagliabue E, Dolci C, Bayssas M. Circulating nerve growth factor level changes during oxaliplatin treatment-induced neurotoxicity in the rat. Anticancer Res. 2002;22:4199-4204. [PubMed] |
| 15. | Onizuka S, Kawakami S, Taniguchi K, Fujioka H, Miyashita K. Pancreatic carcinogenesis: apoptosis and angiogenesis. Pancreas. 2004;28:317-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Correa P. The biological model of gastric carcinogenesis. IARC Sci Publ. 2004;157:301-310. [PubMed] |
| 17. | Schrenk D, Schmitz HJ, Bohnenberger S, Wagner B, Wörner W. Tumor promoters as inhibitors of apoptosis in rat hepatocytes. Toxicol Lett. 2004;149:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Kim SJ, Kim JE, Moon IS. Paraquat induces apoptosis of cultured rat cortical cells. Mol Cells. 2004;17:102-107. [PubMed] |
| 19. | Del Bello B, Valentini MA, Comporti M, Maellaro E. Cisplatin-induced apoptosis in melanoma cells: role of caspase-3 and caspase-7 in Apaf-1 proteolytic cleavage and in execution of the degradative phases. Ann N Y Acad Sci. 2003;1010:200-204. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Twiddy D, Brown DG, Adrain C, Jukes R, Martin SJ, Cohen GM, MacFarlane M, Cain K. Pro-apoptotic proteins released from the mitochondria regulate the protein composition and caspase-processing activity of the native Apaf-1/caspase-9 apoptosome complex. J Biol Chem. 2004;279:19665-19682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Cain K. Chemical-induced apoptosis: formation of the Apaf-1 apoptosome. Drug Metab Rev. 2003;35:337-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Del Bello B, Valentini MA, Mangiavacchi P, Comporti M, Maellaro E. Role of caspases-3 and -7 in Apaf-1 proteolytic cleavage and degradation events during cisplatin-induced apoptosis in melanoma cells. Exp Cell Res. 2004;293:302-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Jazirehi AR, Gan XH, De Vos S, Emmanouilides C, Bonavida B. Rituximab (anti-CD20) selectively modifies Bcl-xL and apoptosis protease activating factor-1 (Apaf-1) expression and sensitizes human non-Hodgkin's lymphoma B cell lines to paclitaxel-induced apoptosis. Mol Cancer Ther. 2003;2:1183-1193. [PubMed] |
| 24. | Dong ML, Zhu YC, Hopkins JV. Oil A induces apoptosis of pancreatic cancer cells via caspase activation, redistribution of cell cycle and GADD expression. World J Gastroenterol. 2003;9:2745-2750. [PubMed] |
| 25. | Fu YG, Qu YJ, Wu KC, Zhai HH, Liu ZG, Fan DM. Apoptosis-inducing effect of recombinant Caspase-3 expressed by constructed eukaryotic vector on gastric cancer cell line SGC7901. World J Gastroenterol. 2003;9:1935-1939. [PubMed] |
| 26. | Boulares AH, Ren T. Mechanism of acetaminophen-induced apoptosis in cultured cells: roles of caspase-3, DNA fragmentation factor, and the Ca2+ and Mg2+ endonuclease DNAS1L3. Basic Clin Pharmacol Toxicol. 2004;94:19-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
Edited by Wang XL Proofread by Zhu LH and Xu FM