Published online Sep 1, 2004. doi: 10.3748/wjg.v10.i17.2514
Revised: February 24, 2004
Accepted: March 4, 2004
Published online: September 1, 2004
AIM: Menoease Pills (MP), a Chinese medicine-based new formula for postmenopausal women, has been shown to modulate the endocrine and immune systems[1]. The present study investigated the effects of MP and one of its active ingredients, ligustrazine, on epithelial barrier and ion transport function in a human colonic cell line, T84.
METHODS: Colonic transepithelial electrophysiological characteristics and colonic anion secretion were studied using the short circuit current (ISC) technique. RT-PCR was used to examine the expression of cytoplasmic proteins associated with the tight junctions, ZO-1 (zonula occludens-1) and ZO-2 (zonula occludens-2).
RESULTS: Pretreatment of T84 cells with MP (15 μg/mL) for 72 h significantly increased basal potential difference, transepithelial resistance and basal ISC. RT-PCR results showed that the expressions of ZO-1 and ZO-2 were significantly increased after MP treatment, consistent with improved epithelial barrier function. Results of acute stimulation showed that apical addition of MP produced a concentration-dependent (10-5000 μg/mL, EC50 = 293.9 μg/mL) increase in ISC. MP-induced ISC was inhibited by basolateral treatment with bumetanide (100 μmol/L), an inhibitor of the Na + -K + -2Cl- cotransporter, apical addition of Cl- channel blockers, diphenylamine-2, 2’-dicarboxylic acid (1 mmol/L) or glibenclamide (1 mmol/L), but not 4, 4’-diisothiocyanostilbene-2, 2’-disulfonic acid or epithelial Na + channel blocker, amiloride. The effect of MP on ZO-1 and ZO-2 was mimicked by Ligustrazine and the ligustrazine-induced ISC was also blocked by basolateral application of bumetanide and apical addition of diphenylamine-2, 2'-dicarboxylic acid or glibenclamide, and reduced by a removal of extracellular Cl-.
CONCLUSION: The results of the present study suggest that MP and ligustrazine may improve epithelial barrier function and exert a stimulatory effect on colonic anion secretion, indicating the potential use of MP and its active ingredients for improvement of GI tract host defense and alleviation of constipation often seen in the elderly.
-
Citation: Zhu JX, Yang N, Zhang GH, Tsang LL, Gou YL, Wong HYC, Chung YW, Chan HC. Improvement of barrier function and stimulation of colonic epithelial anion secretion by
Menoease Pills . World J Gastroenterol 2004; 10(17): 2514-2518 - URL: https://www.wjgnet.com/1007-9327/full/v10/i17/2514.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i17.2514
It is well known that the gastrointestinal (GI) epithelium of the host, as the first defense line, plays an important role in protecting enteric epithelia from invasion of most pathogens. Intestinal epithelial barrier function regulates epithelial ions and nutrient transport as well as host defense mechanisms. Epithelial membrane pumps, ion channels and tight junctions tightly control epithelial transcellular and paracellular fluxes[2,3]. Cl- secretion also provides an essential driving force for lubrication of intestinal contents during regular bowel movements or flushing of microbial organisms or artificial irritants in host defense responses[4,5]. Epithelial Cl- channels play an important role in regulation and maintenance of normal GI physiological functions. Abnormal regulation of Cl- channels may result in diarrhea[6-8] or constipation[9,10]. While the later represents one of the frequently encountered conditions in aged people, few remedies are available for alleviation of the condition in the elderly.
Menoease Pills (Modified Bak Foong Pills, MP), a newly developed formula based on traditional Chinese medicine Bak Foong Pills (BFP, also known as Baifeng Wan)[11-17], has been designed for the use of postmenopausal women. It has been demonstrated that MP can regulate hormonal profiles (Gou et al, unpublished data) and immune system in the elderly[1], indicating its beneficial effects for postmenopausal or elderly women. Since our previous studies have demonstrated that BFP could increase colonic epithelial Cl- and pancreatic duct epithelial HCO3- secretion[11,15,16] and both BFP and MP have a common active ingredient, ligustrazine, we undertook the present study to examine whether MP and ligustrazine exerted any effect on Cl- secretion and epithelial electrophysiological characteristics using human colonic T84 cells in conjunction with the short-circuit current technique and RT-PCR.
Dulbecco’s Modified Eagle’s medium (DMEM)/F12, Hank’s balanced salt solution (HBSS), and fetal bovine serum were from Gibco Laboratories (New York, NY). 4, 4’-diisothiocyanostilbene-2, 2’-disulfonic acid (DIDS) and glibenclamide were from Sigma (St. Louis, MO). MP was obtained from Eu Yan Sang Ltd (Hong Kong). Diphenylamine-2, 2’-dicarboxylic acid (DPC) was purchased from Riedel-de Haen Chemicals (Hannover, Germany). Calbiochem (San Diego, CA) was the source for amiloride hydrochloride and bumetanide. Krebs-Henseit (K-H) solution had the following composition (mmol/L) : NaCl, 117; KCl, 4.5; CaCl2, 2.5; MgCl2, 1.2; NaHCO3, 24.8; KH2PO4, 1.2; glucose, 11.1. The solution was gassed with 950 mL/L O2 and 50 mL/L CO2, at pH7.4.
Five hundred gram of MP powder in 700 mL/L ethanol at a ratio of 1 to 10 (g/mL) was put in round-bottomed flask and boiled under reflux for 2 h . The mixture was filtered and the residues of MP were subject to the same treatment for a second time. The filtrates from the two treatment procedures were collected and put in the vacuum rotary evaporator for concentration. The extracts were collected and lyophilized by a freeze dryer.
Human colonic T84 cells were purchased from American Type Culture Collection (Rockville, MD). The cells were grown in DMEM/F12 with 100 mL/L fetal bovine serum. For ISC recording the cells (2-3 × 105/mL) were plated onto each floating permeable support, which was made of a Millipore filter with a silicone rubber ring attached on top of it for confining the cells (culture area 0.45 cm2). For the RT-PCR analysis, cells were seeded on the Millipore filter with a confined culture area of 4.5 cm2. Cultures were incubated at 37 °C in 950 mL/L O2 and 50 mL/L CO2 for 6 d before experiments. For the experiments of MP and ligustrazine pretreatments, MP (15 μg/mL) or ligustrazine (100 μmol/L) was added into the culture medium at 72 h before experiments, when the cells became semi-confluent.
The measurement of ISC has been described previously[18]. Monolayers grown on permeable supports were clamped vertically between two halves of the Ussing chamber. The monolayers were bathed in both sides with Krebs-Henseit solution, which was maintained at 37°C by a water jacket enclosing the reservoir. The Krebs-Henseit solution was bubbled with 950 mL/L O2 and 50 mL/L CO2 to maintain the pHof the solution at 7.4. Drugs could be added directly to apical or basal side of the epithelium. Usually, the epithelia exhibited a basal transepithelial potential difference for every monolayer examined, which was measured by the Ag/AgCl reference electrodes (World Precision Instruction) connected to a preamplifier which was in turn connected to a voltage-clamp amplifier (World Precision Instruction, DVC-1000). In most of the experiments, the change in ISC was defined as the maximal rise in ISC following agonist stimulation and it was normalized to current change per unit area of the epithelial monolayer (μA/cm2). The total charges transported for 15 min (the area under the curve of the agonist-induced ISC responses) were also used to describe the agonist-induced responses (μC/cm2). In each experiment, a transepithelial potential difference was 0.1 mV. The change in current in response to the applied potential was used to calculate the transepithelial resistance (TER) of the monolayer using Ohm’s Law. Experiments were normally repeated in different batches of culture to ensure that the data were reproducible.
Total RNA (15 μg) was extracted from the T84 (control, MP and ligustrazine pretreated). Expressions of ZO-1 and ZO-2 were analyzed by competitive RT-PCR. The specific oligo nucleotide primers for ZO-1 was CGGTCCTCTGAGCCTGTAAG for sense and GGA TCTACATGCGACGACAA for antisense corresponding to nucleotides 3100-3470 with an expected cDNA of 371 bp[19], and for ZO-2 was GCCAAAACCCAGAACAAAGA for sense and ACTGCTCTCTCCCACCTCCT for antisense corresponding to nucleotides 3018-3283 with an expected cDNA of 212 bp[19]. GAPDH was used as an internal marker for semi-quantitative analysis of expressions of ZO-1 and ZO-2 of T84 cells. The specific oligonucleotide primers for GAPDH were TCC CAT CAC CAT CTT CCA G for sense and TCC ACC ACT GAC ACG TTG for antisense corresponding to nucleotides 249-764 bp with an expected cDNA of 515 bp[20].
Results were expressed as mean ± SD. The number of experiments represents independent measurements on separate monolayers. Comparisons between groups of data were made by Student’s t-test. A P value less than 0.05 was considered statistically significant. EC50 values were determined by nonlinear regression using GraphPad Prism software.
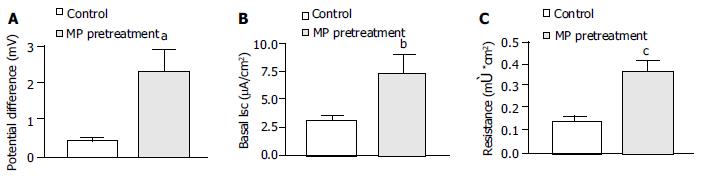
Pretreatment of T84 cells with MP 15 μg/mL (n = 15) for 72 h significantly increased the basal transepithelial potential difference from 0.39 ± 0.07 to 2.27 ± 0.59 mV (Figure 1A, P < 0.01), basal ISC from 3.05 ± 0.44 to 7.14 ± 1.80 μA/cm2 (Figure 1B, P < 0.05) and transepithelial resistance (TER) from 0.14 ± 0.01 to 0.37 ± 0.04 μC/cm2 (Figure 1C, P < 0.001).
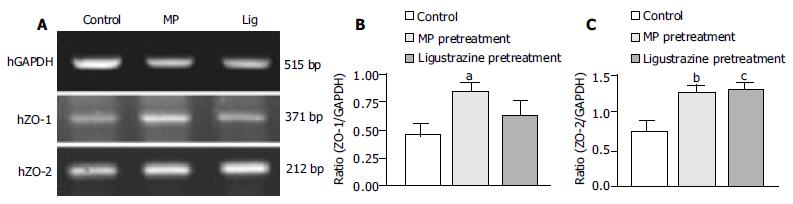
In order to see weather MP-induced TER increase was related to the cytoplasmic proteins associated with tight junctions, ZO-1 (zonula occludens-1) and ZO-2 (zonula occludens-2), we used RT-PCR analysis to examine the expression levels of ZO-1 and ZO-2 in T84 cells (Figure 2A). Semi-quantitative analyses showed that the expression levels of both ZO-1 and ZO-2 after MP pretreatment were significantly elevated, the ratio of ZO-1 to GAPDH was from 0.46 ± 0.08 to 0.81 ± 0.10 (n = 6, P < 0.05, Figure 2B), and the ratio of ZO-2 to GAPDH was from 0.76 ± 0.12 to 1.27 ± 0.12 (n = 4, P < 0.001, Figure 2C), indicating the enhancement of epithelial barrier function.
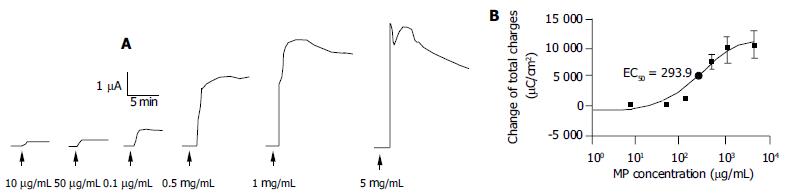
As shown in Figure 3, apical addition of MP (10-5000 μg/mL) produced an ISC increase which was concentration-dependent (Figure 3A) with an apparent EC50 of about 293.9 μg/mL (Figure 3B). MP-induced changes in ISC were calculated as total charges transported for 15 min (μC/cm2, the area under the curve of the MP-induced ISC responses for the given time period) since the current kinetics did not sustain. MP at 10, 50, 100, 500, 1000 and 5000 μg/mL produced ISC increases of 306.7 ± 25.5 (n = 4), 673.3 ± 91.3 (n = 4), 1380.0 ± 119.4 (n = 4), 7624.0 ± 309.7 (n = 5), 9580.0 ± 734.9 (n = 6) and 10053.3 ± 979.1 μC/cm2 (n = 4), respectively.
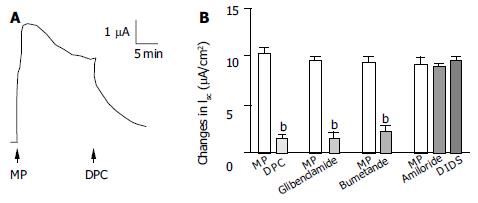
In order to study the ion species involved in mediating MP-induced ISC, a Na + channel blocker, amiloride and a couple of Cl- channel blockers, DPC, glibenclamide and DIDS were examined (Figure 4). The change in ISC was defined as the maximal rise in ISC following MP stimulation and it was normalized to current change per unit area of the epithelial monolayer (μA/cm2). DPC (1 mmol/L, n = 4, Figure 4A) or glibenclamide (1 mmol/L, n = 5) added to the apical side reduced MP (500 μg/mL) -induced responses from 10.0 ± 0.97 μA/cm2 to 1.78 ± 0.18 μA/cm2 (P < 0.01) or from 9.44 ± 0.49 μA/cm 2 to 1.39 ± 0.5 μA/cm2 (P < 0.001) respectively, but apical addition of amiloride (10 μmol/L, n = 4) or DIDS (100 μmol/L, n = 4) had no significant effects (Figure 4B). Basolateral addition of bumetanide (100 μmol/L, n = 6), a strong inhibitor of the Na + -K + -2Cl- cotransporter reduced the MP-induced ISC from 9.33 ± 0.64 to 2.31 ± 0.74 μA/cm2 (Figure 4B, P < 0.01).
Similar to the effects of pretreatment with MP, treating T84 cells with ligustrazine, one of the active ingredients of MP, for 72 h also increased the levels of ZO-1 and ZO-2, the ratio of ZO-1 to GAPDH was raised from 0.46 ± 0.08 to 0.65 ± 0.11 (n = 6, Figure 2B) and the ratio of ZO-2 to GAPDH was from 0.76 ± 0.12 to 1.33 ± 0.07 (n = 4, P < 0.001) (Figure 2C).
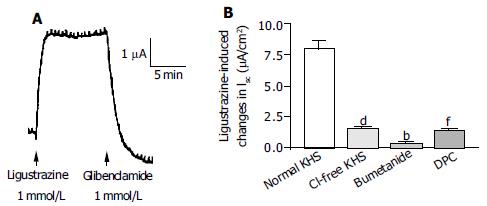
Acute stimulation with ligustrazine (1 mmol/L, apical side) produced a current increase which was similar to that induced by acute addition of MP (0.5 mg/mL, apical) (n = 6, Figure 5A). Removal of Cl- from KHS (n = 4), apical addition of DPC or glibenclamide (1 mmol/L) (n = 3) and basolateral administration of bumetanide (100 mmol/L) (n = 3) reduced ligustrazine-induced current increases by 79.9% (P < 0.001), 82.4% (P < 0.001) and 96.2% (P < 0.001), respectively (Figure 5B).
The present study has provided scientific evidence for the pharmacological action of MP, a Chinese medicine-based formula for postmenopausal women, on the GI tract. The results demonstrated that MP could stimulate Cl- secretion in human colonic epithelial cell line T84. The supporting evidence includes: MP-induced responses were insensitive to Na + channel blockers; the response was inhibited by Cl- channel blockers; and substantially inhibited by the Na + -K + -2Cl- cotransporter inhibitors. The stimulatory effects of MP on colonic anion secretion were mimicked by its active ingredient, ligustrazine. Since ligustrazine is an active ingredient common in both MP and BFP, a traditional formula previously shown to stimulate anion secretion by GI tract epithelia[11,15,16], the present results suggest that Ligustrazine may be one of the responsible ingredients involved in mediating the secretory effects of both MP and BFP.
Apart from its acute stimulatory effects on colonic anion secretion, MP, by treating T84 cells for 72 h, was also demonstrated to significantly alter the electrophysiological characteristics of the colonic epithelia. Increases in transepithelial potential and basal ISC may represent an increased driving force for anion secretion and basal secretion, respectively. These results indicate long-term treatment of MP can promote colonic anion secretion, consistent with its acute effects. On the other hand, pretreatment of T84 cells with MP also increased the transepithelial resistance, indicating its effect on improving epithelial barrier function. This was confirmed by RT-PCR results, which showed that pretreatment with MP significantly up-regulated gene expressions of tight junction related proteins, ZO-1 and ZO-2. Similar results were obtained using Ligustrazine, suggesting that ligustrazine was able to improve barrier function in addition to colonic secretion. It has been reported that an elevation of intracellular calcium could decrease the tight junction resistance in T84 monolayers[21]. Since Ligustrazine has been shown to decrease intracellular Ca2 + by inhibiting Ca2 + entry and/or Ca2 + release[22,23], ligustrazine as well as ligustrazine-containing MP may strengthen tight junctions, thereby enhancing transepithelial resistance. In fact, we have found that intracellular calcium could also be reduced by an apical addition of MP (data not shown), indicating a possible mechanism for improving barrier function. Further studies may be required to understand the detailed mechanisms.
Taken together, the present results have demonstrated that MP and Ligustrazine exert a stimulatory effect on gastrointestinal Cl- secretion and improvement of epithelial barrier function. Since MP is designed for postmenopausal or elderly women, its demonstrated effects on the colonic epithelia, in addition to its beneficial effect on endocrine (Gou et al, unpublished data) and immune systems previously shown[1], suggest that MP and its active ingredient, ligustrazine, may be used to alleviate some of the GI tract disorders, such as infection and constipation, often seen in the elderly.
| 1. | Ho AL, Gou YL, Rowlands DK, Chung YW, Chan HC. Effects of Bak Foong Pills and Menoease Pills on white blood cell distribution in old age female rats. Biol Pharm Bull. 2003;26:1748-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Baumgart DC, Dignass AU. Intestinal barrier function. Curr Opin Clin Nutr Metab Care. 2002;5:685-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 174] [Article Influence: 7.3] [Reference Citation Analysis (2)] |
| 3. | Balkovetz DF, Katz J. Bacterial invasion by a paracellular route: divide and conquer. Microbes Infect. 2003;5:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Hosoda Y, Winarto A, Iwanaga T, Kuwahara A. Mode of action of ANG II on ion transport in guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol. 2000;278:G625-G634. [PubMed] |
| 5. | Albanese CT, Cardona M, Smith SD, Watkins S, Kurkchubasche AG, Ulman I, Simmons RL, Rowe MI. Role of intestinal mucus in transepithelial passage of bacteria across the intact ileum in vitro. Surgery. 1994;116:76-82. [PubMed] |
| 6. | Forte LR, Thorne PK, Eber SL, Krause WJ, Freeman RH, Francis SH, Corbin JD. Stimulation of intestinal Cl- transport by heat-stable enterotoxin: activation of cAMP-dependent protein kinase by cGMP. Am J Physiol. 1992;263:C607-C615. [PubMed] |
| 7. | Grøndahl ML, Jensen GM, Nielsen CG, Skadhauge E, Olsen JE, Hansen MB. Secretory pathways in Salmonella Typhimurium-induced fluid accumulation in the porcine small intestine. J Med Microbiol. 1998;47:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Lencer WI, Delp C, Neutra MR, Madara JL. Mechanism of cholera toxin action on a polarized human intestinal epithelial cell line: role of vesicular traffic. J Cell Biol. 1992;117:1197-1209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 125] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Tenore A, Fasano A, Gasparini N, Sandomenico ML, Ferrara A, Di Carlo A, Guandalini S. Thyroxine effect on intestinal Cl-/HCO3- exchange in hypo- and hyperthyroid rats. J Endocrinol. 1996;151:431-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Ewe K. Intestinal transport in constipation and diarrhoea. Pharmacology. 1988;36 Suppl 1:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Tang N, Zhu JX, Zhao WC, Xing Y, Gou YL, Rowlands DK, Chung YW, Chan HC. Effect of Bak Foong pills on exocrine pancreatic-bile secretion. Biol Pharm Bull. 2003;26:1384-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 12. | Zhou Q, Rowlands DK, Gou YL, Tsang LL, Chung YW, Chan HC. Cardiovascular protective effects of traditional Chinese medicine bak foong pills in spontaneously hypertensive rats. Biol Pharm Bull. 2003;26:1095-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Liu B, Dong XL, Xie JX, Gou YL, Rowlands DK, Chan HC. Effect of Bak Foong pills on enhancing dopamine release from the amygdala of female rats. Biol Pharm Bull. 2003;26:1028-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Gou YL, Ho AL, Rowlands DK, Chung YW, Chan HC. Effects of Bak Foong Pill on blood coagulation and platelet aggregation. Biol Pharm Bull. 2003;26:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Zhu JX, Chan YM, Tsang LL, Chan LN, Zhou Q, Zhou CX, Chan HC. Cellular signaling mechanisms underlying pharmacological action of Bak Foong Pills on gastrointestinal secretion. Jpn J Physiol. 2002;52:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Zhu JX, Lo PS, Zhao WC, Tang N, Zhou Q, Rowlands DK, Gou YL, Chung YW, Chan HC. Bak Foong Pills stimulate anion secretion across normal and cystic fibrosis pancreatic duct epithelia. Cell Biol Int. 2002;26:1011-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Rowlands DK, Tsang LL, Cui YG, Chung YW, Chan LN, Liu CQ, James T, Chan HC. Upregulation of cystic fibrosis transmembrane conductance regulator expression by oestrogen and Bak Foong Pill in mouse uteri. Cell Biol Int. 2001;25:1033-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Ussing HH, Zerahn K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Reprinted from Acta. Physiol. Scand. 23: 110-127, 1951. J Am Soc Nephrol. 1999;10:2056-2065. [PubMed] |
| 19. | Youakim A, Ahdieh M. Interferon-gamma decreases barrier function in T84 cells by reducing ZO-1 levels and disrupting apical actin. Am J Physiol. 1999;276:G1279-G1288. [PubMed] |
| 20. | Usui T, Hara M, Satoh H, Moriyama N, Kagaya H, Amano S, Oshika T, Ishii Y, Ibaraki N, Hara C. Molecular basis of ocular abnormalities associated with proximal renal tubular acidosis. J Clin Invest. 2001;108:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Tai YH, Flick J, Levine SA, Madara JL, Sharp GW, Donowitz M. Regulation of tight junction resistance in T84 monolayers by elevation in intracellular Ca2 + : a protein kinase C effect. J Membr Biol. 1996;149:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Pang PK, Shan JJ, Chiu KW. Tetramethylpyrazine, a calcium antagonist. Planta Med. 1996;62:431-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Liu SY, Sylvester DM. Antiplatelet activity of tetramethylpyrazine. Thromb Res. 1994;75:51-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
Edited by Zhu LH and Xu FM