Published online Aug 15, 2004. doi: 10.3748/wjg.v10.i16.2334
Revised: April 1, 2004
Accepted: April 15, 2004
Published online: August 15, 2004
AIM: To investigate the relations between tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and Helicobacter pylori (H pylori) infection in apoptosis of gastric epithelial cells and to assess the expression of TRAIL on the surface of infiltrating T-cells in H pylori-infected gastric mucosa.
METHODS: Human gastric epithelial cell lines and primary gastric epithelial cells were co-cultured with H pyloriin vitro, then recombinant TRAIL proteins were added to the culture. Apoptosis of gastric epithelial cells was determined by a specific ELISA for cell death. Infiltrating lymphocytes were isolated from H pylori-infected gastric mucosa, and expression of TRAIL in T cells was analyzed by flow cytometry.
RESULTS: The apoptosis of gastric epithelial cell lines and primary human gastric epithelial cells was mildly increased by interaction with either TRAIL or H pylori alone. Interestingly, the apoptotic indices were markedly elevated when gastric epithelial cells were incubated with both TRAIL and H pylori (Control vs TRAIL and H pylori: 0.51 ± 0.06 vs 2.29 ± 0.27, P = 0.018). A soluble TRAIL receptor (DR4-Fc) could specifically block the TRAIL-mediated apoptosis. Further studies demonstrated that infiltrating T-cells in gastric mucosa expressed TRAIL on their surfaces, and the induction of TRAIL sensitivity by H pylori was dependent upon direct cell contact of viable bacteria, but not CagA and VacA of H pylori.
CONCLUSION: H pylori can sensitize human gastric epithelial cells and enhance susceptibility to TRAIL-mediated apoptosis. Modulation of host cell sensitivity to apoptosis by bacterial interaction adds a new dimension to the immunopathogenesis of H pylori infection.
-
Citation: Wu YY, Tsai HF, Lin WC, Chou AH, Chen HT, Yang JC, Hsu PI, Hsu PN.
Helicobacter pylori enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in human gastric epithelial cells. World J Gastroenterol 2004; 10(16): 2334-2339 - URL: https://www.wjgnet.com/1007-9327/full/v10/i16/2334.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i16.2334
Helicobacter pylori (H pylori), which infects about 50% of the world’s population, is the leading cause of chronic gastritis and peptic ulcer diseases. Recent studies have shown that apoptosis of gastric epithelial cells is increased during H pylori infection[1-4]. The enhanced gastric epithelial cell apoptosis in H pylori infection has been suggested to play an important role in the pathogenesis of chronic gastritis, peptic ulcer and gastric neoplasia. There are a number of mechanisms that may be involved, including the direct cytotoxic effects of the bacteria, as well as inflammatory responses elicited by the infection[4-7]. Recent studies have suggested that T helper type 1 (Th1) cells are selectively increased during H pylori infection[8-11]. Th1 cytokines, such as gamma interferon (IFN-) and tumor necrosis factor alpha (TNF-), can increase the release of proinflammatory cytokines, augmenting apoptosis induced by H pylori[7]. H pylori infection could also induce gastric mucosa damage by increasing expression of Fas in gastric epithelial cells, leading to gastric epithelial cell apoptosis through Fas/FasL interaction with infiltrating T cells[6,12]. These findings suggest a role for immune-mediated apoptosis of gastric epithelial cells during H pylori infection.
TRAIL, a novel TNF superfamily member with strong homology to FasL, is capable of inducing apoptosis in a variety of transformed cell lines in vitro[13,14]. Recent studies indicated that TRAIL-induced apoptosis occurred through a caspase signaling cascade[15-17]. It has been shown that T cells can kill target cells via TRAIL/TRAIL receptor interaction[18-23], suggesting that TRAIL might serve as a cytotoxic effector molecule in activated T cells in vivo. In addition to its role in inducing apoptosis by binding to death receptors, TRAIL itself can stimulate T cells and augment IFN- secretion[24]. These findings have led us to hypothesize that TRAIL/TRAIL receptor interaction was involved in the apoptosis of gastric epithelial cells in H pylori gastritis. We therefore designed the study to investigate the interactions between TRAIL and H pylori in apoptosis of human gastric epithelial cells. Additionally, we assessed the expression of TRAIL on the surface of infiltrating T-cells in H pylori-infected gastric mucosa.
H pylori standard strain ATCC43504 (CagA + , VacA + ) and the mutant strain ATCC51932 (CagA - , VacA - ), obtained from American Type Culture Collections (ATCC) were used. H pylori strain NTUH-C1 (CagA + , VacA + ) was isolated from a patient with duodenal ulcer in National Taiwan University Hospital. Before each experiment, H pylori was passaged on 5% sheep blood agar plates by incubation in an atmosphere consisting of 5% O2, 15% CO2, and 80% N2 for 2-4 d at 37 °C. Bacteria were cultured in Brucella broth (Difco Labs, Inc. Detroit, MI) supplemented with 5% FBS, vancomycin and amphotericin B under the same conditions for 30 h at 37 °C with agitation (80-100 rpm/min). Cells were then pelleted and resuspended in phosphate-buffered saline at a concentration of 108 CFU/mL. Human gastric adenocarcinoma cell line AGS was obtained from ATCC and maintained in DMEM, supplemented with 10% FBS.
For generation of H pylori isogenic mutant strains with cagA or vacA gene defect, we introduced mutations into cagA or vacA gene in H pylori strain NTUH-C1. cagA and vacA genes were amplified by PCR with the following primer pairs, cagA: 5’-ATGACTAACGAAACTATTGATCAAACA-3’ and 5’-AGATTTTTGGAAACCACCTT TTGTATTA-3’; vacA: 5’-GCTGGGATTGGGGGAATG-3’ and 5’-TTGCGCGCTA TTGGGTGG-3’. The PCR fragments were purified and then cloned on pGEM-T easy vector by a TA cloning kit (Promega, Madison, WI, USA). The cloned cagA or vacA gene was digested with Nhe I, after which the chloramphenicol resistant marker (CAT cassette) was ligated with recombinant Nhe I sites at both ends. The plasmid pGEM-T Easy Vector with cagA or vacA inserted by the CAT cassette was used for the natural transformation of H pylori strain NTUH-C1. The cagA gene or vacA gene isogenic knockout mutants were selected by the BHI agar plate with 40 ppm chloramphenicol and confirmed with PCR.
Recombinant TRAIL proteins were expressed in E. coli expression system and purified with Ni column as described[24]. In brief, the coding portion of the extracellular domain of TRAIL (amino acid 123-314) was PCR amplified, subcloned into pRSET B vector (Invitrogen, Groningen, the Netherlands) and expressed in E. coli expression system. Purification of recombinant His-TRAIL fusion protein was performed by metal chelate column chromatography using Ni-NTA resin according to the manufacturer’s recommendations (Qiagen, Hilden, Germany). His-TRAIL was quantified by the Bradford method and protein assay reagent (BioRad, Richmond, CA, USA). To generate soluble recombinant DR4-Fc fusion molecules, the coding sequence for the extracellular domain of human DR4 was isolated by RT-PCR using the forward primer, CGGATTTCATGGCGCCACCACCA, and the reverse primer, GAAGATCTATTATGTCCATTGCC. The amplified product was ligated in-frame into BamHI-cut pUC19-IgG1-Fc vector containing the human IgG1 Fc coding sequence. The fusion gene was then subcloned into pBacPAK9 vector (Clontech, Palo Alto, CA). DR4-Fc fusion protein was recovered from the filtered supernatants of the recombinant virus-infected Sf21 cells using protein G-sepharose beads (Pharmacia, Piscataway, NJ). The bound DR4-Fc protein was eluted with glycine buffer (pH 3) and dialyzed into PBS. The DR4-Fc is a soluble TRAIL receptor, which could bind to TRAIL and block its effect.
The culture of human gastric epithelial cells was adapted from the methods described by Smoot et al[25]. Gastric biopsies were obtained from patients undergoing gastric endoscopy in National Taiwan University Hospital for dyspepsia with informed consents. Specimens were collected in Leibowitz’s L-15 medium (Life Technologies, Grand Island, NY). Gastric cells were isolated enzymatically after mechanically mincing the tissue, into pieces less than or equal to 1 mm in size. The tissue was then pelleted by centrifugation at 1500 rpm for five minutes at 4 °C, the collagenase/dispase was discarded, then the tissue was washed once in 10 mL of PBS and pelleted again by centrifugation. The cells were resuspended in the cell culture medium. The gastric epithelial cells obtained above were suspended in 2 mL of Ham’s F12 cell culture medium (Life Technologies, Grand Island, NY) with 10% FBS and placed into a 6-well tissue culture cluster well.
A sensitive ELISA which detects cytoplasmic histone-associated DNA fragments was performed according to the manufacturer’s protocol (Cell Death Detection ELISAPLUS; Roche Mannheim Biochemicals, Mannheim Germany). Human gastric epithelial cells were cultured in a 96-well plate (104 cells/well) overnight, then treated with TRAIL recombinant protein, harvested by centrifugation at 200 g. The cells were lysed by incubation with lysis buffer for 30 min, followed by centrifugation at 200 g for 10 min at room temperature. The supernatant was collected and incubated with immunoreagent pre-prepared for 2 h. After washed gently, the supernatant was pipetted into each well with a substrate solution and kept in the dark until development of the color was sufficient for photometric analysis. The reaction was determined in a spectrophotometer at 405 nm.
AGS cells or primary human gastric epithelial cells 5 × 10 4/well were incubated with H pylori (ATCC strain 43504 or strain 51932) under the concentration of 4 × 10 7 CFU for 12 h. Recombinant TRAIL proteins were added into the culture at the concentration of 1.0 g/mL for 4 h in the absence or presence of soluble TRAIL receptor, DR4-Fc (30 g/mL), which could block the function of TRAIL proteins. Apoptosis of gastric epithelial cells was determined by a specific ELISA as described.
To exclude the possibility that the effect of induction of TRAIL sensitivity in AGS cells by H pylori was a general phenomenon in Gram-negative bacteria, possibly via interaction with LPS on cell wall, we further studied the TRAIL-mediated apoptosis in AGS cells following contact with LPS and Campylobacter jejuni. AGS cells were incubated with LPS from E. coli, Campylobacter jejuni, and H pylori (domestic strain NTUH-C1) for 12 h. Recombinant TRAIL proteins were added into the culture. Apoptosis was measured with ELISA as described.
AGS cells were incubated with H pylori (ATCC strain 43504; CagA + , VacA + ) concentrated culture supernatant or with live bacteria separated by a membrane filter (Nunc Tissue Culture; Nunc, Roskilde, Denmark) under the concentration of 4 × 10 7 CFU/5 × 10 4 cells for 12 h. Recombinant TRAIL proteins were added into the culture. Cell death was measured with ELISA as described.
AGS cells (5 × 10 4 cells) were incubated with a CagA-negative and VacA-negative H pylori mutant strain (ATCC strain 51932) as well as the cagA or vacA gene defect NTUH-C1 isogenic mutant strains for 12 h. Recombinant TRAIL proteins were then added into the culture. Apoptosis was measured with ELISA assay as described.
For isolation of gastric infiltrating lymphocytes from endoscopic biopsy specimens, the collected tissues were immediately placed in ice-cold RPMI 1640 complete medium (GIBCO BRL, Gaithersburg, MD, USA) containing 10% fetal calf serum (FCS) supplemented with penicillin (50 IU/mL), streptomycin (50 mg/mL), L-glutamine (2 mmol/L) and sodium pyruvate (1 mmol/L). The tissues were washed, diced into 1 mm3 pieces and treated with collagenase (Type I, 5 g/mL; Sigma Co. St. Louis, MO) and heparin (5 U/mL) in RPMI 1640 complete medium at 37 °C for 60 min. After the samples were washed twice with RPMI 1640 medium, mononuclear cells were collected and then stained with anti-CD3 and anti-TRAIL mAb. The expression of TRAIL on the surface of T cells was detected by flow cytometry according to our previous study[24].
The values of apoptosis assay were presented as mean ± SD. The results were compared by Student’s t test. A P value less than 0.05 was regarded as statistically significant.
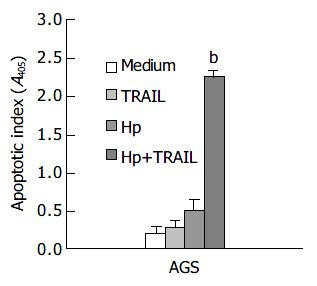
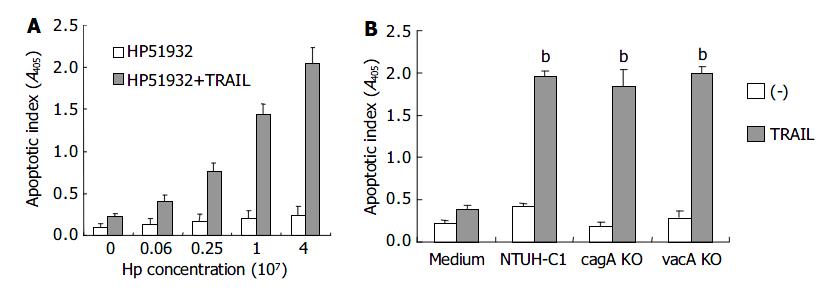
Figure 1 shows the effects of H pylori and TRAIL on apoptosis in AGS cells. The apoptosis of AGS cells was slightly increased by TRAIL proteins or H pylori alone. Interestingly, the apoptotic index was dramatically increased when AGS cells were incubated with both TRAIL and H pylori (P < 0.01 as compared with control medium, TRAIL or H pylori alone). Further studies disclosed that TRAIL-induced apoptosis in AGS cells was increased by H pylori in a dose-dependent manner (data not shown).
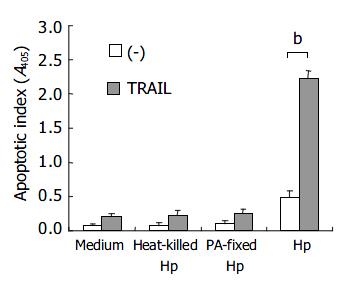
Figure 2 shows that the TRAIL-induced apoptosis depended on viable H pylori because exposure of the host cells to heat-killed H pylori and paraformaldehyde-fixed H pylori did not induce sensitivity to TRAIL-mediated apoptosis.
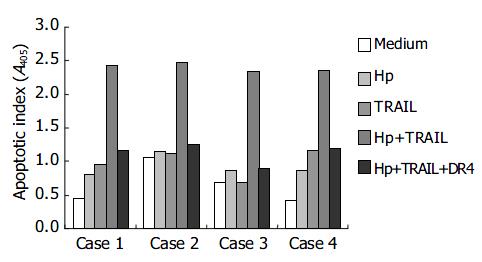
We further isolated and cultured primary human gastric epithelial cells from 23 different human cases (H pylori-positive: 12 cases; H pylori-negative: 11 cases) to investigate the effects of H pylori and TRAIL on apoptosis. The apoptosis of primary human gastric epithelial cells was mildly elevated by either TRAIL or H pylori alone (Table 1). However, the apoptotic indices were markedly elevated when the primary gastric epithelial cells were incubated with both H pylori and TRAIL. The apoptosis induced by H pylori and TRAIL was specifically blocked by adding soluble TRAIL receptor, DR4-Fc, indicating that the cell death was resulted from interaction between TRAIL and TRAIL receptor on the cell surface. Figure 3 shows the representative responses of four cases. All the gastric epithelial cells tested were sensitive to TRAIL after interaction with H pylori in vitro.
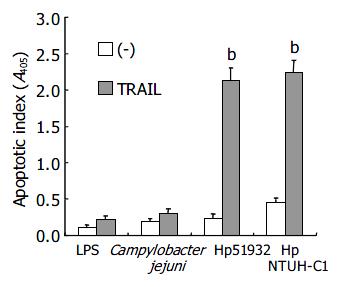
To exclude the possibility that the effect of induction of TRAIL sensitivity by H pylori was a general phenomenon in Gram-negative bacteria, possible via interaction with lipopolysaccharide (LPS) on cell walls, we further studied the induction of sensitivity to TRAIL-mediated apoptosis in AGS cells after interaction with LPS from E. coli and with Campylobacter jejuni, a Gram-negative bacterial pathogen in gastrointestinal tract. The results in Figure 4 demonstrate that H pylori enhanced TRAIL-mediated apoptosis in AGS cells. However, neither LPS from E. coli nor Campylobacter jejuni was able to induce TRAIL-sensitivity. Moreover, similar to ATCC strains, domestic H pylori strain NTUH-C1 was also capable of inducing TRAIL-sensitivity in AGS cells (Figure 4). These results indicated the ability to sensitize human gastric epithelial cells to TRAIL-induced apoptosis was H pylori-specific and not a general phenomenon in Gram-negative bacteria.
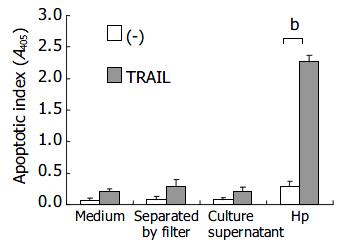
To investigate the effects of direct bacteria-epithelial cell contact in induction of TRAIL sensitivity by H pylori, AGS cells and bacteria were separated by a membrane filter (Nunc Tissue Culture; Nunc, Roskilde, Denmark). Figure 5 shows the effects of direct cell contact of bacteria on TRAIL-mediated apoptosis in human gastric epithelial cells. The enhancement of TRAIL sensitivity by H pylori was abrogated when AGS cells were separated by the filter without direct bacteria-cell contact or co-cultured with concentrated H pylori culture supernatant without viable bacteria, indicating that direct contact with viable bacteria was required for induction of TRAIL sensitivity by H pylori.
To exclude the possibility that the death of gastric epithelial cells induced by TRAIL was related with vacA cytotoxin of H pylori, and to elucidate the role of cagA gene products in onset of the apoptotic process, we examined the abilities to enhance TRAIL-mediated apoptosis in H pylori mutant strain, ATCC 51932, which is deficient of cytotoxin vacA and cagA, and H pylori NTUH-C1 cag A, vacA gene knock out isogenic mutant strains. Figure 6 shows the effects of CagA-negative and VacA-negative H pylori strains on TRAIL-mediated apoptosis in gastric epithelial cells. H pylori strain 51932 significantly enhanced sensitivity to TRAIL-mediated apoptosis in gastric epithelial cell lines in a dose-dependent manner. cagA or vacA gene knock out NTUH-C1 mutant strain was also capable of inducing TRAIL sensitivity in gastric epithelial cells. Taken together, these results suggested that vac A and cagA proteins of H pylori were not required for induction of TRAIL-sensitivity in human gastric epithelial cells.
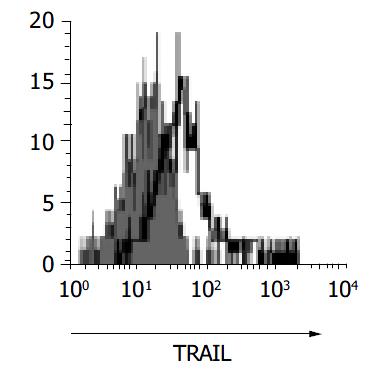
To confirm the expression of TRAIL in gastric infiltrating lymphocytes, gastric biopsy specimens were collected from subjects and examined by flow cytometry. Figure 7 demonstrates the expression of TRAIL on surface of T-lymphocytes isolated from gastric mucosa. Flow cytometry confirmed the presence of TRAIL on the surface of gastric infiltrating T-lymphocytes.
In this study, we investigated the effects of H pylori and TRAIL on apoptosis in human gastric epithelial cells. The apoptosis of primary human gastric epithelial cells was mildly increased after incubated with either H pylori or TRAIL alone. Interestingly, the apoptotic indices of gastric epithelial cells were markedly increased when the primary gastric epithelial cells were incubated with both TRAIL and H pylori. The induction of TRAIL-mediated apoptosis by H pylori was specifically blocked by adding soluble TRAIL receptor, DR4-Fc. The results implicated that H pylori could directly trigger apoptosis in gastric epithelial cells as previously described[26-28] Additionally, the microorganism was also capable of enhancing TRAIL sensitivity in gastric epithelial cells. Most of the apoptosis in gastric epithelial cells after exposure to both H pylori and TRAIL was resulted from interaction between TRAIL and TRAIL receptor on the surface of gastric epithelial cells, but not due to direct cytotoxicity induced by H pylori.
To confirm the expression of TRAIL in infiltrating T-lymphocytes of gastric mucosa, we isolated the gastric infiltrating lymphocytes from gastric biopsy specimens of H pylori-infected subjects. Our data confirmed that TRAIL was expressed on the surface of gastric infiltrating T-cells. This important finding further supports the hypothesis that TRAIL/ TRAIL-R interaction is involved in epithelial cell damage during H pylori infection. Furthermore, it implicates that infiltrating T-cells in gastric mucosa may play a crucial role in the sequential changes of H pylori-related chronic gastritis.
In this study, we also demonstrated that neither Campylobacter jejuni nor LPS from E. coli was able to induce TRAIL-sensitivity in AGS cells. In contrast, H pylori standard strain ATCC43504 (CagA + , VacA + ), mutant strain ATCC51932 (CagA - , VacA - ) and our domestic strain NTUH-C1 (CagA + , VacA + ) were capable of inducing TRAIL-sensitivity in AGS cells. The results indicate the ability to enhance TRAIL sensitivity of gastric epithelial cells is specific for H pylori and not a general phenomenon of Gram-negative Bacteria.
It has been demonstrated recently that H pylori could directly trigger cell death by cytotoxins after interaction with gastric epithelial cells[26,27,29]. Other reports have shown that H pylori could secrete cagA into gastric epithelial cells by Type IV secretion, thus inducing intracellular protein phosphorylation in host cells[30-33]. To elucidate the role of cagA gene products and vacA in TRAIL-mediated apoptosis, we examined the ability to induce TRAIL of a CagA-negative and vacA-negative H pylori mutant strain, ATCC 51932 as well as cagA, vacA gene knock out mutants of NTUH-C1 strain. All the H pylori wild type and mutant strains significantly enhanced sensitivity to TRAIL-mediated apoptosis in gastric epithelial cell lines. These findings suggest that expression of cagA and vacA is not mandatory for induction of TRAIL-sensitivity in gastric epithelial cells.
To investigate the effects of direct bacteria-epithelial cell contact in induction of TRAIL sensitivity by H pylori, AGS cells were separated by the filter without direct bacteria-cell contact or co-cultured with concentrated H pylori culture supernatant without viable bacteria. The enhanced TRAIL sensitivity was only observed when AGS cells were co-cultured with viable H pylori, but not with bacteria culture supernatant or with viable bacteria separated by a permeable membrane, indicating that direct contact with viable bacteria is necessary for induction of TRAIL sensitivity of gastric epithelial cells.
We demonstrated here that human gastric epithelial cells sensitized to H pylori conferred susceptibility to TRAIL-mediated apoptosis. Although the induction of TRAIL sensitivity by H pylori in gastric epithelial cells was independent of H pylori virulent factors CagA and VacA, the degree of apoptosis was linked to the presence of H pylori and the associated inflammatory response. Therefore, the degree of mucosal damage was also determined by the inflammatory response induced by H pylori within gastric epithelium. Our results suggest a role for immune-mediated apoptosis of gastric epithelial cells by infiltrating T cells during Helicobacter infection.
In conclusion, H pylori enhance susceptibility of gastric epithelial cells to TRAIL-mediated apoptosis. The induction of TRAIL sensitivity by H pylori is dependent upon direct contact of viable bacteria with gastric epithelial cells, but independent of expression of H pylori virulent factors vacA and cagA. Gastric infiltrating T-lymphocytes can express TRAIL on their cell surfaces. Modulation of host cell apoptosis by bacterial interaction adds a new dimension to the immune pathogenesis in chronic Helicobacter infection.
We thank Dr. N Sutkowski for the critical review of the manuscript, Dr. SB Wang and JM Wong (Department of Medicine, National Taiwan University Hospital) for supporting endoscopic biopsy specimens, Ms. WI Tsai and WH Wu for assistance with H pylori cultures.
| 1. | Jones NL, Shannon PT, Cutz E, Yeger H, Sherman PM. Increase in proliferation and apoptosis of gastric epithelial cells early in the natural history of Helicobacter pylori infection. Am J Pathol. 1997;151:1695-1703. [PubMed] |
| 2. | Mannick EE, Bravo LE, Zarama G, Realpe JL, Zhang XJ, Ruiz B, Fontham ET, Mera R, Miller MJ, Correa P. Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res. 1996;56:3238-3243. [PubMed] |
| 3. | Moss SF, Calam J, Agarwal B, Wang S, Holt PR. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut. 1996;38:498-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 315] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 4. | Rudi J, Kuck D, Strand S, von Herbay A, Mariani SM, Krammer PH, Galle PR, Stremmel W. Involvement of the CD95 (APO-1/Fas) receptor and ligand system in Helicobacter pylori-induced gastric epithelial apoptosis. J Clin Invest. 1998;102:1506-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 180] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | Fan X, Crowe SE, Behar S, Gunasena H, Ye G, Haeberle H, Van Houten N, Gourley WK, Ernst PB, Reyes VE. The effect of class II major histocompatibility complex expression on adherence of Helicobacter pylori and induction of apoptosis in gastric epithelial cells: a mechanism for T helper cell type 1-mediated damage. J Exp Med. 1998;187:1659-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 147] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Jones NL, Day AS, Jennings HA, Sherman PM. Helicobacter pylori induces gastric epithelial cell apoptosis in association with increased Fas receptor expression. Infect Immun. 1999;67:4237-4242. [PubMed] |
| 7. | Wagner S, Beil W, Westermann J, Logan RP, Bock CT, Trautwein C, Bleck JS, Manns MP. Regulation of gastric epithelial cell growth by Helicobacter pylori: offdence for a major role of apoptosis. Gastroenterology. 1997;113:1836-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 212] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 8. | Bamford KB, Fan X, Crowe SE, Leary JF, Gourley WK, Luthra GK, Brooks EG, Graham DY, Reyes VE, Ernst PB. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 430] [Article Influence: 15.4] [Reference Citation Analysis (1)] |
| 9. | D'Elios MM, Manghetti M, De Carli M, Costa F, Baldari CT, Burroni D, Telford JL, Romagnani S, Del Prete G. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J Immunol. 1997;158:962-967. [PubMed] |
| 10. | Karttunen R, Karttunen T, Ekre HP, MacDonald TT. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut. 1995;36:341-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 190] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Lindholm C, Quiding-Järbrink M, Lönroth H, Hamlet A, Svennerholm AM. Local cytokine response in Helicobacter pylori-infected subjects. Infect Immun. 1998;66:5964-5971. [PubMed] |
| 12. | Wang J, Fan X, Lindholm C, Bennett M, O'Connoll J, Shanahan F, Brooks EG, Reyes VE, Ernst PB. Helicobacter pylori modulates lymphoepithelial cell interactions leading to epithelial cell damage through Fas/Fas ligand interactions. Infect Immun. 2000;68:4303-4311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 69] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2187] [Cited by in RCA: 2212] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 14. | Griffith TS, Lynch DH. TRAIL: a molecule with multiple receptors and control mechanisms. Curr Opin Immunol. 1998;10:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 348] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 15. | Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood WI. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1275] [Cited by in RCA: 1296] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 16. | Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1163] [Cited by in RCA: 1167] [Article Influence: 40.2] [Reference Citation Analysis (1)] |
| 17. | LeBlanc H, Lawrence D, Varfolomeev E, Totpal K, Morlan J, Schow P, Fong S, Schwall R, Sinicropi D, Ashkenazi A. Tumor-cell resistance to death receptor--induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med. 2002;8:274-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 391] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 18. | Kayagaki N, Yamaguchi N, Nakayama M, Eto H, Okumura K, Yagita H. Type I interferons (IFNs) regulate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression on human T cells: A novel mechanism for the antitumor effects of type I IFNs. J Exp Med. 1999;189:1451-1460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 369] [Cited by in RCA: 360] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 19. | Kayagaki N, Yamaguchi N, Nakayama M, Kawasaki A, Akiba H, Okumura K, Yagita H. Involvement of TNF-related apoptosis-inducing ligand in human CD4+ T cell-mediated cytotoxicity. J Immunol. 1999;162:2639-2647. [PubMed] |
| 20. | Thomas WD, Hersey P. TNF-related apoptosis-inducing ligand (TRAIL) induces apoptosis in Fas ligand-resistant melanoma cells and mediates CD4 T cell killing of target cells. J Immunol. 1998;161:2195-2200. [PubMed] |
| 21. | Nieda M, Nicol A, Koezuka Y, Kikuchi A, Lapteva N, Tanaka Y, Tokunaga K, Suzuki K, Kayagaki N, Yagita H. TRAIL expression by activated human CD4(+)V alpha 24NKT cells induces in vitro and in vivo apoptosis of human acute myeloid leukemia cells. Blood. 2001;97:2067-2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 119] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Kaplan MJ, Ray D, Mo RR, Yung RL, Richardson BC. TRAIL (Apo2 ligand) and TWEAK (Apo3 ligand) mediate CD4+ T cell killing of antigen-presenting macrophages. J Immunol. 2000;164:2897-2904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 96] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Dörr J, Waiczies S, Wendling U, Seeger B, Zipp F. Induction of TRAIL-mediated glioma cell death by human T cells. J Neuroimmunol. 2002;122:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Chou AH, Tsai HF, Lin LL, Hsieh SL, Hsu PI, Hsu PN. Enhanced proliferation and increased IFN-gamma production in T cells by signal transduced through TNF-related apoptosis-inducing ligand. J Immunol. 2001;167:1347-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Smoot DT, Sewchand J, Young K, Desbordes BC, Allen CR, Naab T. A method for establishing primary cultures of human gastric epithelial cells. Methods Cell Sci. 2000;22:133-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Kuck D, Kolmerer B, Iking-Konert C, Krammer PH, Stremmel W, Rudi J. Vacuolating cytotoxin of Helicobacter pylori induces apoptosis in the human gastric epithelial cell line AGS. Infect Immun. 2001;69:5080-5087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 139] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Le'Negrate G, Ricci V, Hofman V, Mograbi B, Hofman P, Rossi B. Epithelial intestinal cell apoptosis induced by Helicobacter pylori depends on expression of the cag pathogenicity island phenotype. Infect Immun. 2001;69:5001-5009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Domek MJ, Netzer P, Prins B, Nguyen T, Liang D, Wyle FA, Warner A. Helicobacter pylori induces apoptosis in human epithelial gastric cells by stress activated protein kinase pathway. Helicobacter. 2001;6:110-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Cover TL, Krishna US, Israel DA, Peek RM. Induction of gastric epithelial cell apoptosis by Helicobacter pylori vacuolating cytotoxin. Cancer Res. 2003;63:951-957. [PubMed] |
| 30. | Odenbreit S, Püls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 954] [Cited by in RCA: 975] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 31. | Asahi M, Azuma T, Ito S, Ito Y, Suto H, Nagai Y, Tsubokawa M, Tohyama Y, Maeda S, Omata M. Helicobacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells. J Exp Med. 2000;191:593-602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 376] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 32. | Stein M, Rappuoli R, Covacci A. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc Natl Acad Sci U S A. 2000;97:1263-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 451] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 33. | Higashi H, Tsutsumi R, Muto S, Sugiyama T, Azuma T, Asaka M, Hatakeyama M. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science. 2002;295:683-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 790] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
Edited by Wang XL Proofread by Chen WW and Xu FM