Published online Aug 1, 2004. doi: 10.3748/wjg.v10.i15.2284
Revised: April 3, 2004
Accepted: April 9, 2004
Published online: August 1, 2004
AIM: To evaluate the glycated hemoglobin (HbA 1c) determination methods and to determine fructosamine in patients with chronic hepatitis, compensated cirrhosis and in patients with chronic hepatitis treated with ribavirin.
METHODS: HbA1c values were determined in 15 patients with compensated liver cirrhosis and in 20 patients with chronic hepatitis using the ion-exchange high performance liquid chromatography and the immunoassay methods. Fructosamine was determined using nitroblue tetrazolium.
RESULTS: Forty percent of patients with liver cirrhosis had HbA1c results below the non-diabetic reference range by at least one HbA1c method, while fructosamine results were either within the reference range or elevated. Twenty percent of patients with chronic hepatitis (hepatic fibrosis) had HbA1c results below the non -diabetic reference range by at least one HbA1c method. In patients with chronic hepatitis treated with ribavirin, 50% of HbA1c results were below the non-diabetic reference using at least one of the HbA1c methods.
CONCLUSION: Only evaluated in context with all liver function parameters as well as a red blood count including reticulocytes, HbA 1c results should be used in patients with advanced liver disease. HbA 1c and fructosamine measurements should be used with caution when evaluating long-term glucose control in patients with hepatic cirrhosis or in patients with chronic hepatitis and ribavirin treatment.
- Citation: Lahousen T, Hegenbarth K, Ille R, Lipp RW, Krause R, Little RR, Schnedl WJ. Determination of glycated hemoglobin in patients with advanced liver disease. World J Gastroenterol 2004; 10(15): 2284-2286
- URL: https://www.wjgnet.com/1007-9327/full/v10/i15/2284.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i15.2284
Measurement of glycated hemoglobin (HbA1c) is used for routine evaluation and management of patients with diabetes mellitus. Concentrations of HbA1c provide a means of assessing long-term glycemic status and correlate well with development of complications related to diabetes mellitus[1,2]. The liver plays a major role in regulating glucose metabolism because it is the main source of endogenous glucose and a major site involved in insulin metabolism. Because liver disease is associated with an increased prevalence of impaired glucose tolerance and diabetes mellitus, there is a need for tools to measure its long-term glycemic control[3]. Previous studies indicated that both HbA1c and fructosamine measurement should not be used in patients with liver cirrhosis, although the reason for this was unclear[4-6]. Shortened erythrocyte life span as in hemolytic anemia is known to cause clinically and analytically low HbA1c values independent of glycemia[7] , but measurement of fructosamine, which has been used to document glycemic status over a period of 2- 4 wk, should not be affected by erythrocyte life span. This study described the determination of HbA1c and fructosamine as well as parameters of liver disease and anemia in patients with advanced liver disease.
Blood samples were collected, with and without EDTA, from 15 consecutive patients with compensated liver cirrhosis and 20 patients with chronic hepatitis and fibrosis of the liver. Diagnostic liver biopsies were performed routinely in all patients during the course of treatment in the Division of Gastroenterology and Hepatology, Department of Internal Medicine, Medical University in Graz. Liver cirrhosis was histologically defined as a diffuse process characterized by fibrosis and the conversion of normal liver architecture into structurally abnormal nodules[8,9]. Of 15 patients with compensated liver cirrhosis Child-Pugh class A (total bilirubin < 2 mg/dL, serum albumin > 3.5 g/dL, prothrombine time 1-4 s prolonged, no hepatic encephalopathy and no ascites), 6 were tested positive for hepatitis C, 8 had alcoholic liver disease and 1 had primary biliary cirrhosis. Of the 20 patients with chronic hepatitis and fibrosis, 19 were tested positive for hepatitis C and 1 suffered from alcoholic liver disease. Ten of these patients with chronic hepatitis C were treated with interferon-α plus the antiviral drug ribavirin that can cause reversible hemolytic anemia[10] . None of the patients included in the study had a history of impaired glucose tolerance or diabetes mellitus.
HbA1c was measured within 3 d of collection using the Hi-Auto A1c HA-8140 HPLC (Menarini Diagnostics, Florence, Italy), the DCA 2000 immunoassay method (Bayer, Vienna, Austria) and the Roche Cobas Integra immunoassay method (Roche, Vienna, Austria) . Each of these HbA1c methods was certified by the National Glycohemoglobin Standardization Program (NGSP)[11]. Routine hematological data were determined with a Coulter counter (Beckman, Vienna, Austria). Blood glucose was determined with a hexokinase/glucose-6-phosphate dehydrogenase colorimetric method (Gluco-Quant; Roche, Vienna, Austria) and used as mean of 4-6 measurements on separate days during the preceding 1 mo. The relationship of blood glucose and HbA1c was calculated according to MBG (mmol/L) = (1.98·HbA1c) - 4.29[12]. Fructosamine was determined with a colorimetric test that uses nitroblue tetrazolium in alkaline solution (Unimate FRA; Roche, Vienna, Austria). Reference ranges were provided by each manufacturer and in most cases represented the mean ± 2SD of a population without known diabetes. All determinations were analyzed blindly and the procedures were in accordance with the declaration of Helsinki and the local ethics committee recommendations.
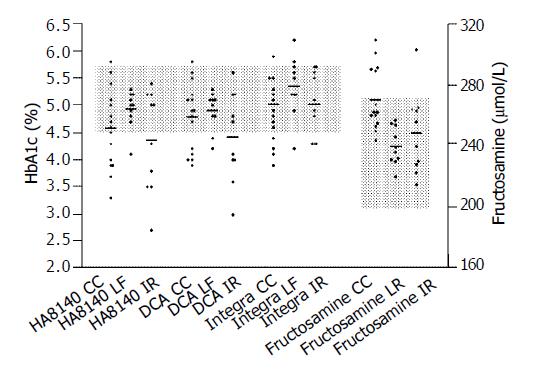
Forty percent (6/15) of the patients with liver cirrhosis had HbA1c levels below the non-diabetic reference range with at least one HbA1c method, while fructosamine concentrations were either within the reference range (n = 10) or elevated (n = 5) (Figure 1). Twenty percent (2/10) of the patients with chronic hepatitis had HbA1c levels below the non-diabetic reference range with at least one HbA1c method. Fructosamine concentrations of all the 10 patients with chronic hepatitis were below the non-diabetic reference range. In patients with chronic hepatitis treated with ribavirin, 50%(5/10) of HbA1c levels were below the non-diabetic reference range detected by at least one of the HbA1c methods (Figure 1). One patient in this group demonstrated a fructosamine concentration within the diabetic range.
Table 1 shows the percentage of patients in each group (cirrhosis, chronic hepatitis, chronic hepatitis with interferon and ribavirin treatment) that the levels of erythrocyte, hematocrit and hemoglobin were below the normal range, and reticulocyte counts above the normal range. Although 30-53% of the patients with cirrhosis and chronic hepatitis demonstrated moderate anemia, none had a reticulocyte count within normal. All of those with low HbA1c also demonstrated anemia but some patients with anemia did not have low HbA1c. Seventy to eighty percent of the patients with chronic hepatitis treated with ribavirin demonstrated moderate anemia and 30% also had high reticulocyte counts (Table 1). All of those with high reticulocyte counts, as well as some of those with anemia and normal reticulocyte counts, had below-normal HbA1c. This study showed elevated reticulocytes, which might be a sign of shortened erythrocyte life span, in only 3 patients with chronic hepatitis and ribavirin treatment. In these patients HbA1c was below the non-diabetic reference range on all methods. We also found HbA1c values below the non-diabetic reference range in up to 40% of the patients with liver cirrhosis and in 50% of the patients with chronic hepatitis treated with ribavirin as measured by at least one of the HbA1c methods. In these groups of patients the HbA1c levels were negatively correlated to the percentage of reticulocytes (Pearson correlation, r = -0.55 to -0.79 depending on method, P < 0,05 for all methods). There was no significant relationship between HbA1c and reticulocyte count in the patients with chronic hepatitis and no ribavirin therapy.
| Group | Patients below normal (%) (Reference range) | Patients above normal (%)Reticulocytes (5-20%) | ||
| Erythrocytes (4.5-5.9 T/L) | Hct (40-50%) | Hb (13-17 g/dL) | ||
| Cirrhosis (n = 15) | 46 | 53 | 40 | 0 |
| Chronic hepatitis (n = 10) | 30 | 30 | 30 | 0 |
| Chronic hepatitis /ribavirin (n = 10) | 80 | 80 | 70 | 30 |
We performed an one- sample t-test comparing mean blood glucose calculated of HbA1c results (MBG (mmol/L) = (1.98·HbA1c) -4.29) and measured blood glucose as the actual value. HbA1c results of the HPLC Menarini HA-8140 and the immunoassay method DCA 2000 were used to calculate a desired blood glucose value because in Pearson correlation they did not correlate with blood glucose. In patients with chronic hepatitis treated with ribavirin the one sample t-test for measured blood glucose and calculated blood glucose resulted in a significant difference (t9Menarini= 7.68, P < 0.05; t9DCA = 6.67, P < 0.05). In patients with liver cirrhosis calculated blood glucose was up to 1 mmol/L lower than measured blood glucose but a high standard deviation (Table 2) caused no statistical difference.
| Measured MBG (mmol/L) | Menarini HA-8140 Calculated MBG (mmol/L) | DCA 2000 Bayer Calculated MBG (mmol/L) | |
| Cirrhosis (n = 15) | 5.8 ± 1.9 | 4.8 | 5.1 |
| Chronic hepatitis (n = 10) | 5.1 ± 0.3 | 5.4 | 5.4 |
| Chronic hepatitis /Ribavirin (n = 10) | 5.2 ± 0.4 | 4.3 | 4.5 |
No correlation was found for all 3 groups between HbA1c results and hepatic serum parameters as -glutamyl transferase (GGT), glutamate-oxalate transaminase (GOT) and glutamyl-pyruvic transaminase (GPT). In all 3 patient groups total protein measured in serum was within normal and albumin was normal in all patients with chronic hepatitis. Three patients with compensated cirrhosis had serum albumin below normal. There was no correlation found in all 3 patient groups between fructosamine results and total protein or albumin. In patients with hepatic cirrhosis, mean fructosamine was within the high non-diabetic reference range. In patients with chronic hepatitis, fructosamine was close to the middle of the non-diabetic reference range. Five patients with cirrhosis and one patient with chronic hepatitis treated with ribavirin had high fructosamine levels even though they had normal blood glucose values.
The liver plays a major role in regulating glucose metabolism because it is the main source of endogenous glucose and a major site involved in insulin metabolism. The most common pathogenic agents in liver disease are alcohol abuse and infectious hepatitis that may cause disturbed erythropoiesis and decreased red cell survival. Macrocytic anemia is a common feature in liver disease but is still incompletely understood[13]. The antiviral drug ribavirin has been widely used in combination with interferon in the treatment of chronic hepatitis C and a major side effect of ribavirin is a reversible hemolytic anemia[10].
Glycated hemoglobin (GHb) measured as HbA1c in diabetic patients, is used for evaluating long-term control of diabetes mellitus. GHb is the result of irreversible non-enzymatic glycation at one or both NH 2-terminal valines of the hemoglobin’s -chain. The extent of glycation and the relative involvement of the hemoglobin’s -and -chains still remain unclear. Depending on the determination method used the concentration of HbA1c is approximately 4-6% in healthy non-diabetic patients. Glycated hemoglobin most accurately reflects the previous 2-3 mo of glycemic control. Diabetic patients could present with abnormal liver chemistries, representing findings from benign hepatic steatosis to severe cirrhosis of the liver. Some medications to treat diabetes mellitus have an effect on liver metabolism or could even cause hepatotoxic reactions. Liver cirrhosis promotes glucose intolerance and diabetes through various mechanisms including insulin resistance and impaired insulin secretion. Sixty to 80% of patients with liver disease have glucose intolerance and 10-15% eventually develop overt diabetes.
In this study we demonstrated HbA1c values below the non- diabetic reference range in up to 40% of the patients with liver cirrhosis while fructosamine results were either within the reference range or elevated in the diabetic range. However, protein metabolism was normal in our patients and although fructosamine results depend on glycation of serum proteins the results might be altered by reduced hepatic protein synthesis due to impairment of liver function. In 50% of the patients with chronic hepatitis treated with ribavirin, HbA1c values were below the non-diabetic reference range as measured by at least one of the HbA1c methods. In these groups of patients the HbA1c results were negatively correlated to the percentage of reticulocytes that might be caused by disturbed erythropoiesis and decreased red cell survival. In patients with liver cirrhosis and chronic hepatitis treated with ribavirin, the HbA1c calculated value of mean blood glucose was up to 1 mmol/L (18 mg/dL) lower than measured mean blood glucose. This underlines that impairment of liver function has influence on results of HbA1c determination. Fructosamine may be a more reasonable marker for long term glucose control in patients with liver disease, but based on our findings we recommend frequent blood glucose monitoring as a measure for glucose control in patients with advanced liver disease.
We conclude that only evaluated in context with all liver function parameters as well as a red blood count including reticulocytes, HbA1c should be used in patients with liver disease. Although the pathophysiologic reasons have still not been confirmed, our data demonstrate that HbA1c and fructosamine measurements should be used with caution when evaluating long-term glucose control in patients with hepatic cirrhosis or in patients with chronic hepatitis with ribavirin treatment. This interference may be due to alterations in erythrocyte lifespan and altered protein metabolism, but further investigations are needed to elucidate the exact cause of the interference in patients with liver disease.
We kindly acknowledge the determination of HbA1c by the following laboratories: Institute of Chemical and Laboratory Diagnostics, Medical University Graz and Laboratory of the County Hospital in Wagna, Austria. Determination of fructosamine was performed in the Laboratory of the Department of Gynecology, Medical University Graz, Austria.
| 1. | The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17510] [Cited by in RCA: 16447] [Article Influence: 498.4] [Reference Citation Analysis (4)] |
| 2. | Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281:2005-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1765] [Cited by in RCA: 1692] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 3. | Shetty A, Wilson S, Kuo P, Laurin JL, Howell CD, Johnson L, Allen EM. Liver transplantation improves cirrhosis-associated impaired oral glucose tolerance. Transplantation. 2000;69:2451-2454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (2)] |
| 4. | Trenti T, Cristani A, Cioni G, Pentore R, Mussini C, Ventura E. Fructosamine and glycated hemoglobin as indices of glycemic control in patients with liver cirrhosis. Ric Clin Lab. 1990;20:261-267. [PubMed] |
| 5. | Cacciatore L, Cozzolino G, Giardina MG, De Marco F, Sacca L, Esposito P, Francica G, Lonardo A, Matarazzo M, Varriale A. Abnormalities of glucose metabolism induced by liver cirrhosis and glycosylated hemoglobin levels in chronic liver disease. Diabetes Res. 1988;7:185-188. [PubMed] |
| 6. | Nomura Y, Nanjo K, Miyano M, Kikuoka H, Kuriyama S, Maeda M, Miyamura K. Hemoglobin A1 in cirrhosis of the liver. Diabetes Res. 1989;11:177-180. [PubMed] |
| 7. | Jiao Y, Okumiya T, Saibara T, Park K, Sasaki M. Abnormally decreased HbA1c can be assessed with erythrocyte creatine in patients with a shortened erythrocyte age. Diabetes Care. 1998;21:1732-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 1761] [Article Influence: 70.4] [Reference Citation Analysis (1)] |
| 9. | Oberti F, Valsesia E, Pilette C, Rousselet MC, Bedossa P, Aubé C, Gallois Y, Rifflet H, Maïga MY, Penneau-Fontbonne D. Noninvasive diagnosis of hepatic fibrosis or cirrhosis. Gastroenterology. 1997;113:1609-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 272] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 10. | De Franceschi L, Fattovich G, Turrini F, Ayi K, Brugnara C, Manzato F, Noventa F, Stanzial AM, Solero P, Corrocher R. Hemolytic anemia induced by ribavirin therapy in patients with chronic hepatitis C virus infection: role of membrane oxidative damage. Hepatology. 2000;31:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 331] [Article Influence: 12.7] [Reference Citation Analysis (8)] |
| 11. | Little RR, Rohlfing CL, Wiedmeyer HM, Myers GL, Sacks DB, Goldstein DE. The national glycohemoglobin standardization program: a five-year progress report. Clin Chem. 2001;47:1985-1992. [PubMed] |
| 12. | Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA(1c): analysis of glucose profiles and HbA(1c) in the Diabetes Control and Complications Trial. Diabetes Care. 2002;25:275-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 657] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 13. | Maruyama S, Hirayama C, Yamamoto S, Koda M, Udagawa A, Kadowaki Y, Inoue M, Sagayama A, Umeki K. Red blood cell status in alcoholic and non-alcoholic liver disease. J Lab Clin Med. 2001;138:332-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
Edited by Wang XL Proofread by Chen WW and Xu FM