Published online Aug 1, 2004. doi: 10.3748/wjg.v10.i15.2267
Revised: December 23, 2003
Accepted: January 13, 2004
Published online: August 1, 2004
AIM: To study the effect of FR167653 on immunological liver injury (ILI) in mice.
METHODS: ILI was established by tail vein injection of 2.5 mg Bacillus Calmette-Guerin (BCG), and 10 d later with 10 mg lipopolysaccharide (LPS) in 0.2 mL saline (BCG plus LPS). Alanine aminotransferase (ALT), aspartate aminotransferase (AST) in sera and malondialdehyde (MDA), glutathione peroxidase (GSHpx) contents in liver homogenates were assayed by spectrophotometry. The levels of tumor necrosis factor-α (TNF-α ) and nitric oxide (NO) levels in sera were determined using ELISA. Interleukin-1 (IL- 1) produced by peritoneal macrophages was determined by the method of 3 H- infiltrated cell proliferation. The nuclear factor-kappa B (NF-κ B) p65 in liver tissue was analyzed with reverse transcription polymerase chain reaction (RT-PCR) . Liver samples collected were stained with hematoxylin and eosin.
RESULTS: FR167653 (50, 100, 150 mg/kg) could significantly decrease the serum transaminase (ALT, AST) activity and MDA content in liver homogenate, and improve reduced GSHpx level of liver homogenate. Liver histopathological examination showed FR167653 (100, 150 mg/kg) significantly reduced inflammatory cells infiltration and liver cells necrosis. FR167653 (50, 100, 150 mg/kg) significantly lowered TNF- α and NO levels in serum, and IL-1 produced by peritoneal macrophages. Moreover, expression of NF-κ B mRNA in liver tissue of ILI induced by BCG plus LPS was significantly reduced by FR167653.
CONCLUSION: All results showed that FR167653 had significant inhibitory action on ILI in mice.
- Citation: Yao HW, Li J, Chen JQ. FR167653 attenuates murine immunological liver injury. World J Gastroenterol 2004; 10(15): 2267-2271
- URL: https://www.wjgnet.com/1007-9327/full/v10/i15/2267.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i15.2267
It has been demonstrated that tissue inflammation plays a critical role in liver pathology via induction of cellular injury. The infiltration of mononuclear phagocytes into the liver has been shown to correlate with the severity of liver injury. Kupffer cells (KCs) are among the first cells that respond to endotoxins, including lipopolysaccharides (LPS), and are considered to be the primary macrophages involved in the clearance of gut-derived bacteria or bacterial toxins. High portal level of LPS can lead to a pronounced secretion of proinflammatory mediators by KCs and ultimately to endotoxin-induced liver injury[1,2]. Moreover, proinflammatory cytokines such as tumor necrosis factor-α (TNF-α ) and interleukin-1 (IL -1) β have been linked to liver injury[3-5], and shown to be early and important mediators of liver injury [6-8]. Thus, inhibition of KCs activity and proinflammatory cytokines production would be beneficial to alleviate liver injury.
FR167653,1-[7-(4-fluorophenyl)-1,2,3,4-tetrahydro-8-(4-pyridyl) pyrazolo [5,1-c][1,2,4] triazin-2-yl]-2-phenylethanedione sulfate monohydrate, was first discovered to be a potent dual inhibitor of IL-1 and TNF-α production in LPS-stimulated human monocytes and phytohemagglutinin-stimulated human lymphocytes[9]. Takahashi et al[10] and Kawano et al[11] also confirmed the inhibitory effect of FR167653 on cytokine production. FR167653 ameliorated endotoxin shock in rabbits and intravascular coagulation in rats[9,12], and also ameliorated cardiac dysfunction caused by chronic infusion of LPS in rats[13]. Furthermore, FR167653 protected lung, liver, and heart against ischemia-reperfusion injury in dogs[14-17], and the protection against liver ischemia-reperfusion injury was associated with inhibition of proinflammatory cytokines from KCs[18,19]. Recent studies suggest that FR167653 inhibits IL- 1β and TNF-α production via specific inhibition of p38 MAPK activity[12,17,20-22]. Because effective therapy has not been established for liver injury, development of an anti-inflammatory compound is urgently needed. From this viewpoint, it is worthwhile to assess the anti-inflammatory and immunomodulatory effect of FR167653. In the current study, we administered FR167653 to a murine model of Bacillus Calmette Guerin (BCG) and LPS-induced liver injury and assessed histopathological changes and its effects on cytokine production.
Male Kunming strain mice weighing 18-22 g were purchased from Animal Center of Anhui Medical University. Mice were allowed to access to food and tap water ad libitum, and acclimated to facilities for at least 1 wk before any treatments. 1, 1, 3, 1-tetraethoxypropane (TEP) and 5, 5’-dithibis-(2-nitrobenzicacid) (DTNB) were purchased from FLUKA Co., Switzerland. BCG was purchased from Institute of Shanghai Biological Products. ConA, LPS from Escherichia coli, and TNF-α ELISA kit were purchased from Sigma Co., St. Louis, USA. The primers of NF-κ B and β -actin were synthesized by BIOASIA Biotech Co. Shanghai. FR167653 supplied by the Fujisawa Pharmaceutical Co. Ltd. (Tokyo, Japan), was dissolved to 20 mL/L with 5 g/Lmethylcellulose solution. To obtain the dose of 50, 100, or 150 mg/kg body mass (BM), 2.5, 5, or 7.5 µL/g·bm. of 20 mL/L FR167653 solution was injected subcutaneously.
Each mouse was injected with 2.5 mg BCG (viable bacilli) in 0.2 mL saline via tail vein, and 10 d later with 10 mg LPS in 0.2 mL saline. At 4, 8, and 12 h post-injection of LPS, animals received FR167653 at 50, 100, and 150 mg/kg, s.c., respectively. The control group was subcutaneously administered the same volume of 5 g/L methylcellulose solution. The mice were anesthetized with ether, then sacrificed by cervical dislocation 16 h after LPS injection and blood was collected, and centrifuged at 1 500 r/min for 10 min at room temperature. Serum was aspirated and stored at -70 °C until assayed as described below. The liver was also removed and stored at -70 °C until required.
Serum ALT, and AST were determined using commercial kits purchased from Institute of Shanghai Biological Products affiliated to the Ministry of Health.
Livers were thawed, weighed and homogenized with Tris-HCl buffer (5 mmol/L, containing 2 mmol/L EDTA, pH 7.4). Homogenates were centrifuged at 1 000 r/min for 10 min at room temperature and the supernatant was used immediately for the assays of MDA and GSHpx. MDA was measured by the thiobarbituric acid method according to standard techniques. The content of MDA was expressed as nmol per gram liver tissue. GSHpx was measured by the DTNB method, and its content was expressed as U per milligram protein.
Serum TNF-α and NO were measured using commercial kits produced by Sigma Co. and Beijing Biotinge-Tech Co. Ltd, and their levels were expressed as pg/mL and µmol/L respectively. IL-1 produced by peritoneal macrophages was measured according to the method of 3H-infiltrated cell proliferation.
Liver tissue RNA was extracted using RNA easy kit (Invitrogen, USA). To test the efficacy of reverse transcriptase, RT-PCR was performed for β -actin mRNA. Briefly, the first strand of cDNA was synthesized by reverse transcriptase and pooled. The resulting cDNA samples were adjusted to PCR buffer conditions and run for PCR simultaneously. The primers for NF-κ B p65 were 5’- GCG GCC AAG CTT AAG ATC TGC CGA GTA AAC-3’, and 5’-CGC TGC TCT AGA GAA CAC AAT GGC CAC TTG CCG-3’. The primers for β -actin were 5’-TGG AAT CCT GTG GCA TCC ATG AA-3’, and 5’-TAA AAC GCA GCT CAG TAA CAG TC-3’. The amplifications of NF-κ B p65 and β -actin genes are expected to generate 198 bp and 348 bp fragments, respectively. Amplification was performed for 30 cycles, each of which consisted of 1 min of denaturation at 94 °C, 1 min of annealing at 58 °C, and 3 min of primer extension at 72 °C. Ten µL of reaction mixture was loaded to 10 g/L agarose gel containing 0.5 µg/mL ethidium bromide for electrophoresis, the gel was then placed under ultraviolet light for semi-quantitative detection.
Formalin-fixed liver specimens were embedded in paraffin and stained with hematoxylin and eosin for conventional morphological evaluation.
All values were expressed as mean ± SD. The significance of differences between groups was determined by ANOVA followed by Student’s t test. P value of less than 0.05 was considered significant.
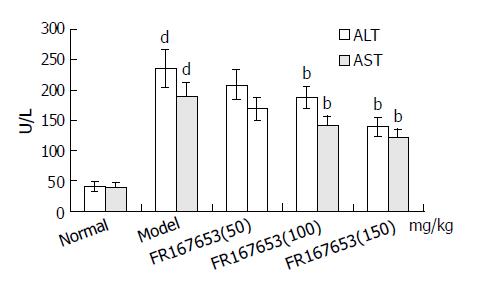
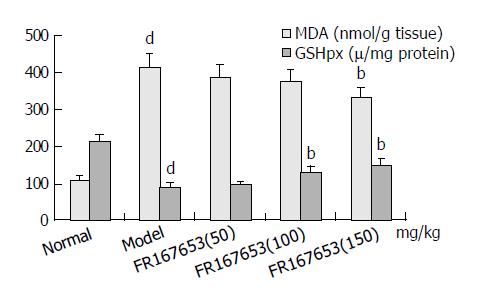
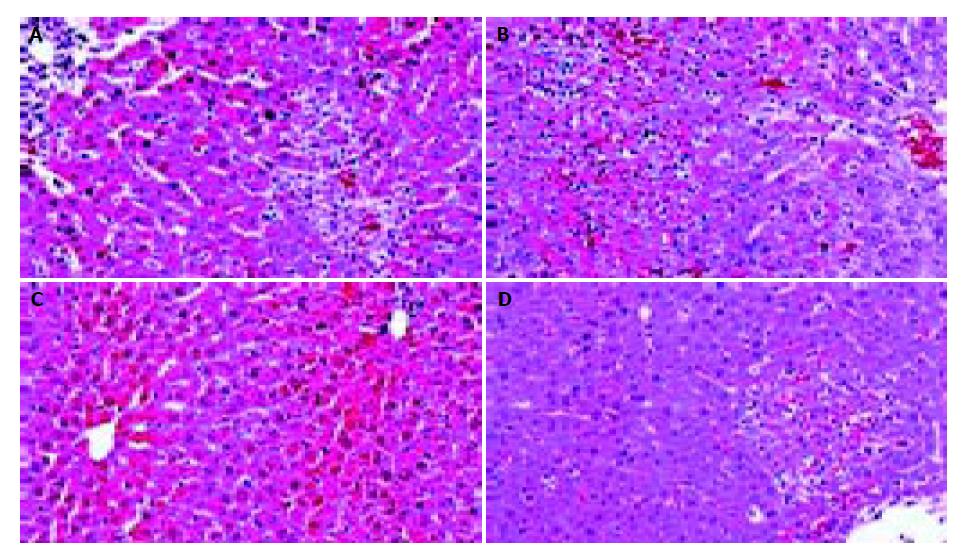
The levels of ALT and AST in plasma and MDA content in liver homogenate were significantly increased after the sequential injection of BCG and LPS. Meanwhile, the GSHpx level in liver homogenate was sharply decreased (Figure 1, Figure 2). FR167653 (50, 100, 150 mg/kg, s.c.) could not only significantly decrease ALT, AST, and MDA levels, but also evidently increase GSHpx level in mice with ILI (Figures 1, 2). Liver histopathologic examination showed extensive inflammatory cells infiltration and liver cells necrosis. FR167653 (100, 150 mg/kg) significantly reduced inflammatory cells infiltration and liver cells necrosis (Figure 3).
The levels of TNF-α and NO were elevated significantly in mice injected with BCG and then challenged with LPS. Likewise, IL-1 excreted by peritoneal macrophages was also significantly increased in the model group (Table 1). FR167653 (50, 100, 150 mg/kg) obviously counteracted the increase of TNF-α and NO levels in sera. IL -1 produced by peritoneal macrophages was also significantly inhibited by FR167653 (Table 1).
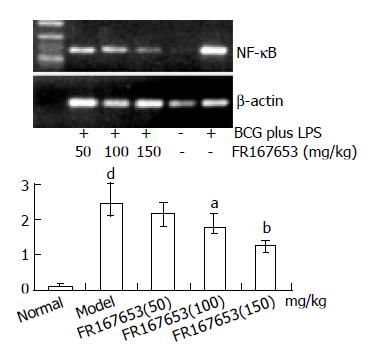
Gel electrophoresis and semi-quantitative analysis showed that FR167653 significantly reduced expression of NF-κ B mRNA in liver tissue of ILI induced by BCG plus LPS compared with model group (Figure 4).
It has been demonstrated that severe hepatitis could be induced by injecting a small dose of bacterial LPS into BCG-pretreated mice[23]. In the present study, ILI was successfully induced by BCG plus LPS. On this basis, FR167653 (50, 100, 150 mg/kg) could significantly lower the increased plasma transaminase levels and MDA contents in liver homogenate. Meanwhile, GSHpx levels were increased significantly. Histopathologic examination showed FR167653 significantly reduced inflammatory cells infiltration and liver cells necrosis.
Although several parameters of the inflammatory response contribute to liver injury[24], one well-studied pathway is the production of TNF-α . In a number of liver injury models, elevated TNF-α levels were present and correlated with liver injury[23,25,26]. Inhibition of TNF-α activity can decrease liver injury. The addition of soluble TNF receptors that diminished the biological effect of TNF-α could significantly decrease liver enzyme levels, improve liver histology, and reduce mortality immediately after acute carbon tetrachloride administration. Similar results were seen in chronic alcohol-induced liver disease in rats. In humans, elevated levels of TNF-α are seen in hepatitis and are associated with increased mortality[27]. Furthermore, TNF-α might act as the first mediator of liver injury, its elevation would result in release of a number of proinflammatory mediators including IL-1, NO, IL-6, IL-8[3,28], further deteriorating the liver injury. In the present study, TNF-α in the sera and IL-1 produced by peritoneal macrophages were significantly increased in mice with ILI compared with control. FR167653, a potent inhibitor of TNF-α and IL-1, suppressed elevated TNF-α and IL-1 levels, which was consistent with previous observations[29]. As potent producers of inflammatory cytokines such as TNF -α and IL-1, KCs have been implicated in the pathway leading to liver injury[30]. Whether inhibition of FR167653 on TNF-α production was associated with diminished KCs proliferation, and decreased inflammatory cells infiltration needs to be further clarified.
A growing body of evidence suggests that sustained production of NO resulting from up-regulation of inducible NOS (iNOS) after LPS challenge may cause hepatocellular injury, either directly[31,32], or indirectly, by forming reactive nitrogen intermediates[33]. Menezes et al[34,35]demonstrated that a NO scavenger, NOX, decreased hepatocellular injury and improved survival after hemorrhagic shock. As reported previously, the synthesis of NO was regulated by various factors including TNF-α , IL-1, and NF-κ B. The inhibition of NF-κ B led to a decrease in the inducible isoform of NO and the transcription of TNF-α in KCs[36-38]. Moreover, synthetic double-stranded oligodeoxynucleotides (ODN) transfer with a high affinity to NF-κ B (NF-κ B/decoy/ODN) in vivo effectively suppressed endotoxin-induced fatal liver injury in mice[39]. In our study, we observed significant suppression of NF-κ B p65 mRNA in liver tissue and NO in sera of BCG plus LPS - induced ILI by FR167653. It indicated that inhibition of FR167653 on liver injury might be related with decreased NO production through inhibiting NF-κ B transcription. Recent studies suggested that p38 MAPK inhibitor, SB203580, diminished phosphorylation of the transactivation domain of the p65 subunit of NF-κ B[40]. Moreover, the pharmacological characteristics and chemical structure of FR167653 resemble SB203580 [41,42]. Therefore, inhibition of FR167653 on liver injury was associated with reduced expression of NF-κ B via p38 MAPK, and led to down-regulation of TNF-α , IL-1, and NO.
| 1. | Enomoto N, Ikejima K, Yamashina S, Hirose M, Shimizu H, Kitamura T, Takei Y, Sato And N, Thurman RG. Kupffer cell sensitization by alcohol involves increased permeability to gut-derived endotoxin. Alcohol Clin Exp Res. 2001;25:51S-54S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Lukkari TA, Järveläinen HA, Oinonen T, Kettunen E, Lindros KO. Short-term ethanol exposure increases the expression of Kupffer cell CD14 receptor and lipopolysaccharide binding protein in rat liver. Alcohol Alcohol. 1999;34:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Shito M, Balis UJ, Tompkins RG, Yarmush ML, Toner M. A fulminant hepatic failure model in the rat: involvement of interleukin-1beta and tumor necrosis factor-alpha. Dig Dis Sci. 2001;46:1700-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Nishioji K, Okanoue T, Mori T, Sakamoto S, Itoh Y. Experimental liver injury induced by Propionibacterium acnes and lipopolysaccharide in macrophage colony stimulating factor-deficient osteopetrotic (op/op) mice. Dig Dis Sci. 1999;44:1975-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Bradham CA, Plümpe J, Manns MP, Brenner DA, Trautwein C. Mechanisms of hepatic toxicity. I. TNF-induced liver injury. Am J Physiol. 1998;275:G387-G392. [PubMed] |
| 6. | Spitzer JA, Zheng M, Kolls JK, Vande Stouwe C, Spitzer JJ. Ethanol and LPS modulate NF-kappaB activation, inducible NO synthase and COX-2 gene expression in rat liver cells in vivo. Front Biosci. 2002;7:a99-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Wang JH, Redmond HP, Wu QD, Bouchier-Hayes D. Nitric oxide mediates hepatocyte injury. Am J Physiol. 1998;275:G1117-G1126. [PubMed] |
| 8. | Hoek JB, Pastorino JG. Ethanol, oxidative stress, and cytokine-induced liver cell injury. Alcohol. 2002;27:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 338] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 9. | Yamamoto N, Sakai F, Yamazaki H, Nakahara K, Okuhara M. Effect of FR167653, a cytokine suppressive agent, on endotoxin-induced disseminated intravascular coagulation. Eur J Pharmacol. 1996;314:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Takahashi S, Shigeta J, Inoue H, Tanabe T, Okabe S. Localization of cyclooxygenase-2 and regulation of its mRNA expression in gastric ulcers in rats. Am J Physiol. 1998;275:G1137-G1145. [PubMed] |
| 11. | Kawano T, Ogushi F, Tani K, Endo T, Ohmoto Y, Hayashi Y, Sone S. Comparison of suppressive effects of a new anti-inflammatory compound, FR167653, on production of PGE2 and inflammatory cytokines, human monocytes, and alveolar macrophages in response to endotoxin. J Leukoc Biol. 1999;65:80-86. [PubMed] |
| 12. | Yamamoto N, Sakai F, Yamazaki H, Sato N, Nakahara K, Okuhara M. FR167653, a dual inhibitor of interleukin-1 and tumor necrosis factor-alpha, ameliorates endotoxin-induced shock. Eur J Pharmacol. 1997;327:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Gardiner SM, Kemp PA, March JE, Bennett T. Influence of FR 167653, an inhibitor of TNF-alpha and IL-1, on the cardiovascular responses to chronic infusion of lipopolysaccharide in conscious rats. J Cardiovasc Pharmacol. 1999;34:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Kamoshita N, Takeyoshi I, Ohwada S, Iino Y, Morishita Y. The effects of FR167653 on pulmonary ischemia-reperfusion injury in dogs. J Heart Lung Transplant. 1997;16:1062-1072. [PubMed] |
| 15. | Kobayashi J, Takeyoshi I, Ohwada S, Iwanami K, Matsumoto K, Muramoto M, Morishita Y. The effects of FR167653 in extended liver resection with ischemia in dogs. Hepatology. 1998;28:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Koyano T, Takeyoshi I, Takahashi T, Hasegawa Y, Sato Y, Ishikawa S, Matsumoto K, Morishita Y. Effect of FR167653 on ischemia-reperfusion injury of the canine heart: ultrastructural study. Transplant Proc. 1998;30:3370-3371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Kobayashi M, Takeyoshi I, Yoshinari D, Matsumoto K, Morishita Y. P38 mitogen-activated protein kinase inhibition attenuates ischemia-reperfusion injury of the rat liver. Surgery. 2002;131:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 85] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Hashimoto K, Nishizaki T, Yoshizumi T, Uchiyama H, Okano S, Ikegami T, Yanaga K, Sugimachi K. Beneficial effect of FR167653 on cold ischemia/reperfusion injury in rat liver transplantation. Transplantation. 2000;70:1318-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Minami S, Furui J, Kanematsu T. Role of carcinoembryonic antigen in the progression of colon cancer cells that express carbohydrate antigen. Cancer Res. 2001;61:2732-2735. [PubMed] |
| 20. | Kobayashi M, Takeyoshi I, Yoshinari D, Koibuchi Y, Koyama T, Kawashima Y, Ohwada S, Matsumoto K, Morishita Y. FR167653 ameliorates ischemia-reperfusion injury of the rat liver through P38 mitogen-activated protein kinase pathway. Transplant Proc. 2001;33:865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Takahashi S, Keto Y, Fujita T, Uchiyama T, Yamamoto A. FR167653, a p38 mitogen-activated protein kinase inhibitor, prevents Helicobacter pylori-induced gastritis in Mongolian gerbils. J Pharmacol Exp Ther. 2001;296:48-56. [PubMed] |
| 22. | Lasky JA, Ortiz LA, Tonthat B, Hoyle GW, Corti M, Athas G, Lungarella G, Brody A, Friedman M. Connective tissue growth factor mRNA expression is upregulated in bleomycin-induced lung fibrosis. Am J Physiol. 1998;275:L365-L371. [PubMed] |
| 23. | Wang GS, Liu GT. Influences of Kupffer cell stimulation and suppression on immunological liver injury in mice. Zhongguo Yao Li Xue Bao. 1997;18:173-176. [PubMed] |
| 24. | Moulin F, Copple BL, Ganey PE, Roth RA. Hepatic and extrahepatic factors critical for liver injury during lipopolysaccharide exposure. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1423-G1431. [PubMed] |
| 25. | Zhang XL, Quan QZ, Sun ZQ, Wang YJ, Jiang XL, Wang D, Li WB. Protective effects of cyclosporine A on T-cell dependent ConA-induced liver injury in Kunming mice. World J Gastroenterol. 2001;7:569-571. [PubMed] |
| 26. | Zang GQ, Zhou XQ, Yu H, Xie Q, Zhao GM, Wang B, Guo Q, Xiang YQ, Liao D. Effect of hepatocyte apoptosis induced by TNF-alpha on acute severe hepatitis in mouse models. World J Gastroenterol. 2000;6:688-692. [PubMed] |
| 27. | Madhotra R, Gilmore IT. Recent developments in the treatment of alcoholic hepatitis. QJM. 2003;96:391-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Muntané J, Rodríguez FJ, Segado O, Quintero A, Lozano JM, Siendones E, Pedraza CA, Delgado M, O'Valle F, García R. TNF-alpha dependent production of inducible nitric oxide is involved in PGE(1) protection against acute liver injury. Gut. 2000;47:553-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Hou Z, Yanaga K, Kamohara Y, Eguchi S, Tsutsumi R, Furui J, Kanematsu T. A new suppressive agent against interleukin-1beta and tumor necrosis factor-alpha enhances liver regen-eration after partial hepatectomy in rats. Hepatol Res. 2003;26:40-46. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Luckey SW, Petersen DR. Activation of Kupffer cells during the course of carbon tetrachloride-induced liver injury and fibrosis in rats. Exp Mol Pathol. 2001;71:226-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Engin A, Bozkurt BS, Altan N, Memiş L, Bukan N. Nitric oxide-mediated liver injury in the presence of experimental bile duct obstruction. World J Surg. 2003;27:253-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Nadler EP, Dickinson EC, Beer-Stolz D, Alber SM, Watkins SC, Pratt DW, Ford HR. Scavenging nitric oxide reduces hepatocellular injury after endotoxin challenge. Am J Physiol Gastrointest Liver Physiol. 2001;281:G173-G181. [PubMed] |
| 33. | Fintelmann S, Jung M, Weidenbach H, Steinle AU, Beger HG, Nüssler AK. [Effect of cellular growth factors on hepatocytes in experimental infection--regulation of NF-kappa B and glutathione homeostasis]. Langenbecks Arch Chir Suppl Kongressbd. 1998;115:405-408. [PubMed] |
| 34. | Menezes J, Hierholzer C, Watkins SC, Lyons V, Peitzman AB, Billiar TR, Tweardy DJ, Harbrecht BG. A novel nitric oxide scavenger decreases liver injury and improves survival after hemorrhagic shock. Am J Physiol. 1999;277:G144-G151. [PubMed] |
| 35. | Hierholzer C, Billiar TR, Tweardy DJ, Harbrecht B. Reduced hepatic transcription factor activation and expression of IL-6 and ICAM-1 after hemorrhage by NO scavenging. Arch Orthop Trauma Surg. 2003;123:55-59. [PubMed] |
| 36. | Shimohashi N, Nakamuta M, Uchimura K, Sugimoto R, Iwamoto H, Enjoji M, Nawata H. Selenoorganic compound, ebselen, inhibits nitric oxide and tumor necrosis factor-alpha production by the modulation of jun-N-terminal kinase and the NF-kappab signaling pathway in rat Kupffer cells. J Cell Biochem. 2000;78:595-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 37. | Cao Q, Mak KM, Lieber CS. Dilinoleoylphosphatidylcholine decreases acetaldehyde-induced TNF-alpha generation in Kupffer cells of ethanol-fed rats. Biochem Biophys Res Commun. 2002;299:459-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Lin M, Rippe RA, Niemelä O, Brittenham G, Tsukamoto H. Role of iron in NF-kappa B activation and cytokine gene expression by rat hepatic macrophages. Am J Physiol. 1997;272:G1355-G1364. [PubMed] |
| 39. | Ogushi I, Iimuro Y, Seki E, Son G, Hirano T, Hada T, Tsutsui H, Nakanishi K, Morishita R, Kaneda Y. Nuclear factor kappa B decoy oligodeoxynucleotides prevent endotoxin-induced fatal liver failure in a murine model. Hepatology. 2003;38:335-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Wilms H, Rosenstiel P, Sievers J, Deuschl G, Zecca L, Lucius R. Activation of microglia by human neuromelanin is NF-kappaB dependent and involves p38 mitogen-activated protein kinase: implications for Parkinson's disease. FASEB J. 2003;17:500-502. [PubMed] |
| 41. | Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, Young PR, Lee JC. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1651] [Cited by in RCA: 1742] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 42. | Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2614] [Cited by in RCA: 2666] [Article Influence: 83.3] [Reference Citation Analysis (5)] |
Edited by Zhu LH Proofread by Chen WW and Xu FM