Published online Aug 1, 2004. doi: 10.3748/wjg.v10.i15.2250
Revised: December 23, 2003
Accepted: January 7, 2004
Published online: August 1, 2004
AIM: Recent studies in both rodents and humans indicated that bone marrow (BM)-derived stem cells were able to home to the liver after they were damaged and demonstrated plasticity in becoming hepatocytes. However, the question remains as to how these stem cells are activated and led to the liver and where the signals initiating the mechanisms of activation and differentiation of stem cells originate. The aim of this study was to investigate the influence of serum from liver-damaged rats on differentiation tendency of bone marrow-derived stem cells.
METHODS: Serum samples were collected from rats treated with a 2-acetylaminofluorene (2-AAF) /carbon tetrachloride (CCl4) program for varying time points and then used as stimulators of cultured BM stem cells. Expression of M2- and L-type isozymes of rat pyruvate kinase, albumin as well as integrin-β 1 were then examined by reverse transcription polymerase chain reaction (RT-PCR) to estimate the differentiation state of BM stem cells.
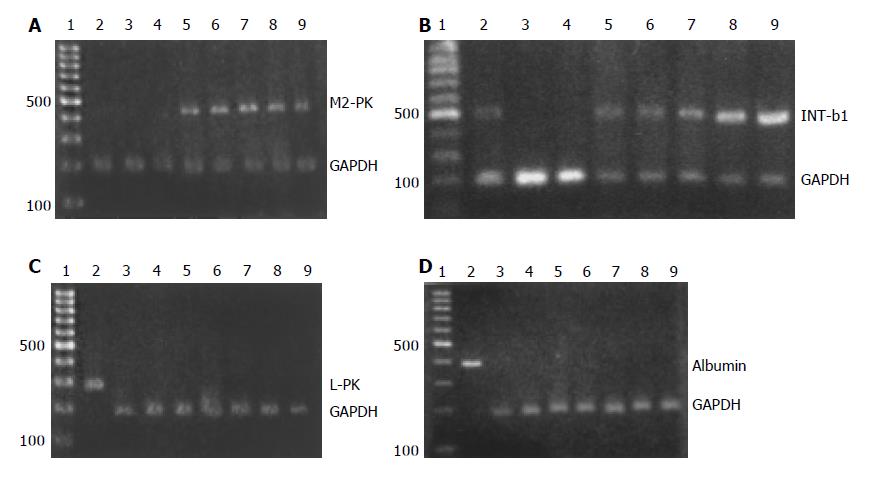
RESULTS: Expression of M2-type isozyme of pyruvate kinase (M2-PK), a marker of immature hepatocytes, was detected in each group stimulated with experimental serum, but not in controls including mature hepatocytes, BM stem cells without serum stimulation, and BM stem cells stimulated with normal control serum. As a marker expressed in the development of liver, the expression signal of integrin-β 1 was also detectable in each group stimulated with experimental serum. However, expression of L-type isozyme of pyruvate kinase (L-PK) and albumin, marker molecules of mature hepatocytes, was not detected in groups stimulated with experimental serum.
CONCLUSION: Under the influence of serum from rats with liver failure, BM stem cells begin to differentiate along a direction to hepatocyte lineage and to possess some features of immature hepatocytes.
- Citation: Hong H, Chen JZ, Zhou F, Xue L, Zhao GQ. Influence of serum from liver-damaged rats on differentiation tendency of bone marrow-derived stem cells. World J Gastroenterol 2004; 10(15): 2250-2253
- URL: https://www.wjgnet.com/1007-9327/full/v10/i15/2250.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i15.2250
Recent studies in both rodents and humans indicated that bone marrow (BM) stem cells were able to home to the liver after they were damaged, and demonstrated plasticity in becoming hepatocytes[1-4]. Questions remain as to how BM stem cells are activated and led to the liver and where the signals initiating the mechanisms of activation and differentiation of stem cells originate. Transfused oval cells (hepatic stem cells) that had a selective tropism for the liver in an animal model of liver-damage suggested that “signal molecules” were present in serum of this animal model and played an important role in mediating both hepatic and non-hepatic stem cell activation[5]. However, the influence of these putative signal molecules in serum on the differentiation state of bone marrow-derived stem cells is yet unclear. The purposes of the present work were to confirm the existence of signaling molecules in serum of liver-damaged rats and to observe its effects on the differentiation of BM stem cells into hepatocytes.
Male Sprague–Dawley (SD) rats, 6-week -old, were used for the establishment of an animal model of liver-damage. The model was made by means of a 2-AAF/CCl4 program according to Petersen[1]. In experimental group, 2-acetylaminofluorene (2 -AAF, Sigma), 2.5 g/L in earthnut oil, was administered to stomach of rats everyday for 7 d. On the 7th d of 2-AAF administration, an Ld50 dose of CCl4 was given by intraperitoneal injection. Animal blood was taken at the time points of 2, 4, 8, 12, and 24 h after CCl4 injection. Experimental serum was prepared on standby. The serum from normal animals was used as control.
The SD rats were sacrificed by means of ether asphyxia. Bone marrow was collected from tibiae and BM cells were suspended in Dulbecco’s modified Eagle’s medium (DMEM) with fetal bovine serum. After centrifugation and re-suspension, the cells were seeded in a culture flask and cultured under a routine condition (37 °C, 50 mL/L CO2). The solution of medium was changed every 4 d while the cells floating on the medium were discarded. The cells adhering to bottom of the flask (so-called BM stromal cells) were cultured sequentially for 12-14 d. After 3 population doublings, the purified cells were harvested and used in the following stimulating culture with experimental serum.
BM stromal cells were cultured sequentially in a specialized medium (DMEM-F12) containing 3 ml/L experimental rat serum. In the control group, the culture medium contained 3 mL/L normal rat serum instead of experimental rat serum. Cultures were grown and submerged for 12 d. The cells were harvested on the 13th culture day for ribonucleic acid (RNA) isolation.
RNA was extracted from the cells collected from the cultures described above, according to the protocol of QIAGEN RNA easy mini kit. RNA samples were then stored at -80 °C.
Genes of M2-type and L-type isozymes of rat pyruvate kinase (M2-PK, L -PK), albumin and integrin-β 1 (INT-β 1) were selected as the markers representing different differentiation stages of hepatocyte lineage. The gene of glyceraldehyde-3-phosphate- dyhydrogenase (GAPDH) was used as an internal control for RT-PCR reactions. Primer pairs used for RT-PCR are shown in Table 1.
| Marker genes | Primer pairs | PCR fragments |
| M2-PK | 5’ccatctaccacttgcagttattcga3’/ 5’tcatggtacaggcactacacgc3’ | 431 bp |
| L-PK | 5’acctctgccttctggatactgact3’/5’tgcaagactccggttcgtatct3’ | 322 bp |
| Albumin | 5’gagcccgaaagaaacgagtgtt3’/5’ggggaatctctggctcatacg3’ | 389 bp |
| INT-β 1 | 5’tacttcagacttccgcattgg3’/ 5’cagtgactgcaaaaatcgtctg3’ | 488 bp |
| GAPDH | 5’ccatggagaaggctggg3’/5’caaagttgtcatggatgacc3’ | 180 bp |
RNA samples were first reversely transcribed into cDNA, and then used as templates in the following PCR reactions. The reaction cocktails (containing 1 µg template cDNA, 50 µmol/L dNTPs, 400 µmol/L primers, 1×PCR buffer, 2.5 mmol/L MgCl2, 1 U Taq-polymerase, add H2O to 50 µL of total volume) were run on GeneAmp® PCR System 9600 (AB) with a combined program of program 1 (at 94 °C for 5 min), program 2 (at 95 °C for 1 min, at 60 °C for 1 min, at 72 °C for 1 min; 30 cycles), and program 3 (at 95 °C for 1 min, at 60 °C for 1 min, at 72 °C for 5 min) . The PCR products were electrophoresed in 12 g/L agarose gel, stained with ethidium bromide, and photographed.
M2-PK, as a marker of immature hepatocytes, was used to estimate the differentiation state of BM stem cells stimulated by the experimental serum. The results showed that the expression signals of M2-PK were detected in each group stimulated with experimental serum, but not in the control group of normal BM stem cells that had no stimulation, BM stem cells stimulated with control serum, and normal mature hepatocytes (Figure 1A). Integrin-β 1 is a marker expressed during the development of liver. In this study, its expression signals were also detectable in each group stimulated with experimental serum as those observed in the positive control of hepatocytes (Figure 1B). L-PK and albumin, as marker molecules of mature hepatocytes, were used to estimate the terminal differentiation state of BM stem cells under the influence of experimental serum. However, no signals were detected in the experimental groups, except for the positive control of hepatocytes (Figure 1C, D).
The existence of liver stem cells had been widely proved in both rodents and humans[1-4,6-9]. By extension, liver stem cells could be divided into three groups: (1) mature hepatocytes that proliferate during normal liver tissue renewal and after less severe liver damage, (2) oval cells that are activated to proliferate when the liver damage is extensive and chronic, and (3) exogenous liver stem cells that may derived from bone marrow cells and respond to severe liver damage[10]. However, in a narrow sense, the concept of liver stem cells is usually limited to hepatic oval cells and non-hepatic bone marrow stem cells.
A phenomenon that was often observed both in experimental animal models and in clinics was that the proliferation of liver stem cells occurred most often in conditions of severe liver damages or chronic liver diseases[11-15]. Maybe it is the reason that stem cells were seldom detected in healthy livers. Thus it can be understood that liver- damage is an important prerequisite for activation of liver stem cells. This suggests that the signals initiating activation of hepatic or non-hepatic stem cells might originate from damaged livers. This hypothesis had been partially proved by our previous experimental work[5]. In our previous experiments, oval cells isolated from male SD rats were transfused, through caudal vein into the circulatory system of a female rat with liver damage. Sex-determining gene sry that was located on Y chromosome was then examined respectively by PCR and in situ hybridization technique in the liver, kidney and spleen of experimental animals. The results of cell-transplant experiments showed that sry gene was detectable only in the liver but not in the spleen and kidney of rats with liver damages and that no signals could be detected in control animals, neither in the liver, spleen nor in the kidney. It could be also morphologically observed that some exogenous cells with sry marker migrated into the parenchyma of liver and settled there, suggesting that transfused oval cells had a selective tropism for damaged liver. These results also suggested that signaling molecules existed in the serum of animals with liver damage and might play a role in mediating stem cell activation.
In the present study, an animal model of liver-damage was established with a 2-AAF/CCl4 program. In this model, the capacity of hepatocyte self-regeneration was first impaired by 2-AAF and then the liver was damaged severely by CCl4. In this status, the damaged liver would likely produce a signal to initiate the activation of stem cells in the bone marrow. The results of the present study showed that the expression of M2-PK, a marker of immature hepatocyte[16-22], could be detected in each group stimulated with experimental serum, but not in any of the control groups. Integrin-β 1 is a marker expressed during the development of liver. Its expression could be detected in fetal hepatocyte as early as at 8th wk of gestation[30]. In the present results, the expression signal of integrin-β 1 was also detectable in each group stimulated with experimental serum. Thus, the functional state of BM-derived stem cells was changed under the influence of experimental serum, thus differentiating toward the direction of a hepatocyte lineage. Although the markers of a mature hepatocyte, L -PK and albumin were not detectable in stimulated BM stem cells, the leap from an undetermined state to a determined state was a marker of entry into the process of programmed differentiation. A variety of possibilities could account for the lack of detectable signals for L-PK and albumin in stimulated BM stem cells. Among the possibilities, one could be the deficiency in intensity and time of stimulation, while another could, by reasoning, be that the postulated “signal molecules” existing in the experimental serum were involved only in the early activation and determination of BM stem cells, while the terminal differentiation of the cells into hepatocytes might still need other signals.
The results in the present study indicated that the driving force promoting differentiation of BM stem cells to hepatocytes was certainly generated from the serum of rats treated by 2-AAF/CCl4. It has been further testified that some “signal molecules” were existed in the circulation of rats treated by 2-AAF/CCl4 and that they might play an important role in the initiation of activation of stem cell. It would be helpful for understanding the mechanisms of stem cell differentiation if the “signal molecules” could be further identified and isolated.
Recent studies have convincingly demonstrated that adult bone marrow contains cells capable of differentiating into hepatocyte-like cells. Nevertheless, what type of cell population are the ancestor cells for hepatocytes still remains a question. In the vast majority of reports, hematopoietic cells were considered to be capable of “transdifferentiating” into hepatocytes[2,4,23-28]. However, Wagers (2002) deemed that there was little evidence for transdifferentiation of adult hematopoietic stem cells[29]. The present study showed that bone marrow stromal cells demonstrated the plasticity in changing into hepatocytes. By this token, the debate about origin of liver stem cells will keep on.
| 1. | Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1795] [Cited by in RCA: 1671] [Article Influence: 61.9] [Reference Citation Analysis (3)] |
| 2. | Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1764] [Cited by in RCA: 1638] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 3. | Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, Henegariu O, Krause DS. Liver from bone marrow in humans. Hepatology. 2000;32:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 900] [Cited by in RCA: 854] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 4. | Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 750] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 5. | Chen JZ, Hong H, Xiang J, Xue L, Zhao GQ. A selective tropism of transfused oval cells for liver. World J Gastroenterol. 2003;9:544-546. [PubMed] |
| 6. | Crosby HA, Hubscher S, Fabris L, Joplin R, Sell S, Kelly D, Strain AJ. Immunolocalization of putative human liver progenitor cells in livers from patients with end-stage primary biliary cirrhosis and sclerosing cholangitis using the monoclonal antibody OV-6. Am J Pathol. 1998;152:771-779. [PubMed] |
| 7. | Lowes KN, Brennan BA, Yeoh GC, Olynyk JK. Oval cell numbers in human chronic liver diseases are directly related to disease severity. Am J Pathol. 1999;154:537-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 338] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 8. | Theise ND, Saxena R, Portmann BC, Thung SN, Yee H, Chiriboga L, Kumar A, Crawford JM. The canals of Hering and hepatic stem cells in humans. Hepatology. 1999;30:1425-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 487] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 9. | Malhi H, Irani AN, Gagandeep S, Gupta S. Isolation of human progenitor liver epithelial cells with extensive replication capacity and differentiation into mature hepatocytes. J Cell Sci. 2002;115:2679-2688. [PubMed] |
| 10. | Sell S. The role of progenitor cells in repair of liver injury and in liver transplantation. Wound Repair Regen. 2001;9:467-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Roskams T, Yang SQ, Koteish A, Durnez A, DeVos R, Huang X, Achten R, Verslype C, Diehl AM. Oxidative stress and oval cell accumulation in mice and humans with alcoholic and nonalcoholic fatty liver disease. Am J Pathol. 2003;163:1301-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 327] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 12. | Lowes KN, Croager EJ, Olynyk JK, Abraham LJ, Yeoh GC. Oval cell-mediated liver regeneration: Role of cytokines and growth factors. J Gastroenterol Hepatol. 2003;18:4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 119] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Fausto N, Campbell JS. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech Dev. 2003;120:117-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 492] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 14. | Oh SH, Hatch HM, Petersen BE. Hepatic oval 'stem' cell in liver regeneration. Semin Cell Dev Biol. 2002;13:405-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Faris RA, Konkin T, Halpert G. Liver stem cells: a potential source of hepatocytes for the treatment of human liver disease. Artif Organs. 2001;25:513-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Tian YW, Smith PG, Yeoh GC. The oval-shaped cell as a candidate for a liver stem cell in embryonic, neonatal and precancerous liver: identification based on morphology and immunohistochemical staining for albumin and pyruvate kinase isoenzyme expression. Histochem Cell Biol. 1997;107:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Tee LB, Kirilak Y, Huang WH, Smith PG, Morgan RH, Yeoh GC. Dual phenotypic expression of hepatocytes and bile ductular markers in developing and preneoplastic rat liver. Carcinogenesis. 1996;17:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Steinberg P, Klingelhöffer A, Schäfer A, Wüst G, Weisse G, Oesch F, Eigenbrodt E. Expression of pyruvate kinase M2 in preneoplastic hepatic foci of N-nitrosomorpholine-treated rats. Virchows Arch. 1999;434:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Hacker HJ, Steinberg P, Bannasch P. Pyruvate kinase isoenzyme shift from L-type to M2-type is a late event in hepatocarcinogenesis induced in rats by a choline-deficient/DL-ethionine-supplemented diet. Carcinogenesis. 1998;19:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Tee LB, Kirilak Y, Huang WH, Morgan RH, Yeoh GC. Differentiation of oval cells into duct-like cells in preneoplastic liver of rats placed on a choline-deficient diet supplemented with ethionine. Carcinogenesis. 1994;15:2747-2756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Scott RJ, English V, Noguchi T, Tanaka T, Yeoh GC. Pyruvate kinase isoenzyme transitions in cultures of fetal rat hepatocytes. Cell Differ Dev. 1988;25:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Wang X, Ge S, McNamara G, Hao QL, Crooks GM, Nolta JA. Albumin-expressing hepatocyte-like cells develop in the livers of immune-deficient mice that received transplants of highly purified human hematopoietic stem cells. Blood. 2003;101:4201-4208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 178] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 24. | Fiegel HC, Lioznov MV, Cortes-Dericks L, Lange C, Kluth D, Fehse B, Zander AR. Liver-specific gene expression in cultured human hematopoietic stem cells. Stem Cells. 2003;21:98-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Austin TW, Lagasse E. Hepatic regeneration from hematopoietic stem cells. Mech Dev. 2003;120:131-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Mallet VO, Mitchell C, Mezey E, Fabre M, Guidotti JE, Renia L, Coulombel L, Kahn A, Gilgenkrantz H. Bone marrow transplantation in mice leads to a minor population of hepatocytes that can be selectively amplified in vivo. Hepatology. 2002;35:799-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 87] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Avital I, Inderbitzin D, Aoki T, Tyan DB, Cohen AH, Ferraresso C, Rozga J, Arnaout WS, Demetriou AA. Isolation, characterization, and transplantation of bone marrow-derived hepatocyte stem cells. Biochem Biophys Res Commun. 2001;288:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 157] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Mitaka T. Hepatic stem cells: from bone marrow cells to hepatocytes. Biochem Biophys Res Commun. 2001;281:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256-2259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1136] [Cited by in RCA: 1028] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 30. | Couvelard A, Bringuier AF, Dauge MC, Nejjari M, Darai E, Benifla JL, Feldmann G, Henin D, Scoazec JY. Expression of integrins during liver organogenesis in humans. Hepatology. 1998;27:839-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 68] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
Edited by Wang XL and Qin D Proofread by Xu FM