Published online Aug 1, 2004. doi: 10.3748/wjg.v10.i15.2157
Revised: December 23, 2003
Accepted: January 8, 2004
Published online: August 1, 2004
Hepatitis E virus (HEV) is an unclassified, small, non-enveloped RNA virus, as a causative agent of acute hepatitis E that is transmitted principally via the fecal-oral route. The virus can cause large water-born epidemics of the disease and sporadic cases as well. Hepatitis E occurs predominantly in developing countries, usually affecting young adults, with a high fatality rate up to 15%-20% in pregnant women. However, no effective treatment currently exists for hepatitis E, and the only cure is prevention. But so far there are no commercial vaccines for hepatitis E available in the world. Although at least four major genotypes of HEV have been identified to date, only one serotype of HEV is recognized. So there is a possibility to produce a broadly protective vaccine. Several studies for the development of an effective vaccine against hepatitis E are in progress and the best candidate at present for a hepatitis E vaccine is a recombinant HEV capsid antigen expressed in insect cells from a baculovirus vector. In this article, the recent advances of hepatitis E and the development of vaccine research for HEV including recombinant protein vaccine, DNA vaccine and the recombinant hepatitis E virus like particles (rHEV VLPs) are briefly reviewed.
- Citation: Wang L, Zhuang H. Hepatitis E: An overview and recent advances in vaccine research. World J Gastroenterol 2004; 10(15): 2157-2162
- URL: https://www.wjgnet.com/1007-9327/full/v10/i15/2157.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i15.2157
Hepatitis E previously known as enterically transmitted non-A, non-B hepatitis, is an infectious viral disease with clinical and epidemiological features of acute hepatitis. It is a water-born disease, transmitted primarily by contaminated water. There is also a possibility of zoonotic spread of the virus, since several non-human primates, pigs, cows, sheep, goats and rodents are susceptible to the infection[1,2]. Hepatitis E virus (HEV) is a principal cause of acute hepatitis in adults throughout much of Asia, Middle East and Northern Africa[3] and transmitted from person-to-person through the fecal- oral route. HEV had provisionally been classified into the caliciviridae family from 1988 to 1998, but now it is classified into the separate genus Hepatitis E-like viruses[4-6] because the phylogenetic analysis of non-structural regions of the virus did not support the classification of HEV into the Caliciviridae family[7]. Although at least four major genotypes have been identified, only one serotype of HEV is recognized[8-10]. HEV infection is endemic in developing countries where sanitary conditions are not well maintained. Over 50 outbreaks have been reported in Southeast and Central Asia, the Middle East, northern and western parts of Africa, and Mexico[11-15]. Most of hepatitis E cases in developed countries have been linked to travel to endemic areas. However, recent studies revealed that hepatitis E also occurred in patients who had never been abroad[16-18]. China is one of the high epidemic areas and there have been 11 hepatitis E epidemics reported to date. The largest one in the world occurred in Xinjiang Uighur Autonomous Region, the Northwest of China, during 1986-1988, with a total number of 119 280 cases and more than 700 deaths[19-20]. Hepatitis E accounts for more than 50% of acute viral hepatitis in young adults of developing countries, although only 1% to 3% of non-pregnant patients progress to fatal fulminant hepatitis, the case-fatality rate can be as high as 20% among pregnant patients[21], constituting a serious public health problem and stressing the need for development of an effective vaccine. The development of an attenuated or killed vaccine is not currently possible because of lacking an efficient cell culture system for replication of HEV[22-27], although some cell lines have been reported for culturing and isolating HEV in vitro[28,29]. Therefore, either a nucleic acid-based vaccine or a recombinant protein vaccine is needed. Compared with gene engineering vaccine for hepatitis B, the study of hepatitis E recombinant vaccine was only a recent endeavor, but some progress has been made[30].
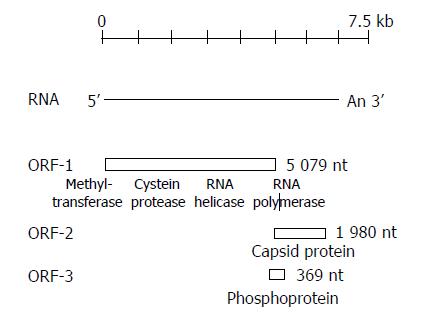
HEV genome consists of a linear, single-stranded, positive-sense RNA of approximately 7.5 kb containing a 3’ poly (A) tail and 3’ noncoding (NC) regions, and contains three overlapping open reading frames (ORFs). All three coding frames are used to express different proteins[31-33] (Figure 1). ORF1 begins at the 5’ end of the viral genome after a 27-bp non-coding sequence and extends 5 079 nt to the 3’ end. ORF1 encodes a polyprotein of about 1 690 amino acids (aa) consisting of non-structural proteins that are involved in viral genome replication and viral protein processing, as its sequence contains motifs characteristic of viral methyltransferases, papain-like cysteineproteases, helicases and RNA-dependent RNA polymerases. In addition, ORF1 has two regions called Y and X domains of unknown function. The very short 5’ NTR at the 5’ end of the viral genome of 27-35 nucleotides is consistent with a capped genome. The ability of a monoclonal antibody to recognize 7-methylguanosine in RNA extracted from virions of different HEV genotypes suggests that HEV RNAs are capped[34,35]. ORF3 is 369 nt long, located at the end of ORF1 and overlaps ORF1 at its 5’ end by only 1 nt, and overlaps ORF2 by 328 nt. It encodes for a 123-aa protein (pORF3), which is expressed intracellularly. The studies of the biology of HEV replication have shown that pORF3 may be capable of associating with the liver cell cytoskeleton and appears to serve as a cytoskeletal anchor site, where pORF2 and RNA can bind to begin the process of viral nucleocapsid assembly[36].
The ORF2 is located between 5147 and 7127 nt, consists of 1980 nt and encodes 660 aa (71 kDa -88 kDa) most likely representing one or more structural or capsid protein(s)[2,29-31]. However, the size of the ORF2 protein in native virons is not known[37]. In vitro assays suggest that the - 88 kDa of glycoprotein is co-translationally translocated across the endoplastic reticulum and is expressed intracellularly as well as on the cell surface[38]. ORF2 contains important epitopes that can induce neutralizing antibodies and has been the focus of vaccine development[39]. Major epitopes appear to exist near the carboxyl ends of ORF2 and ORF3. Epitopes contained in ORF2 are more conserved (90.5%) than epitopes contained in ORF3 (73.5%) in different strains. Many different ORF2 antigens have been shown to induce antibody (Table 1). There are a number of reports suggesting that truncated ORF2 peptide of shorter length might be more antigenic than the full-length protein[40-44]. However, in the majority of cases, it has not been shown that the resulting antibodies are neutralizing and, therefore, it is not known whether these antigens could serve as vaccine candidates. Only three ORF2 antigens (trpE-C2, Burma 62 kDa, Pakistan 55 kDa) thus far have been shown to induce antibodies that neutralize the virus[33].
| HEV (origin) | Designation | Amino acid | |
| N’ | C’ | ||
| Expressed in E. coil | |||
| China | ORF2 | 1 | 660 |
| Burma | TrpE-C2 | 221 | 660 |
| Burma | SG3 | 328 | 654 |
| China | ORF2.1 | 394 | 660 |
| Mexico, Burma | 3.2 | 612 | 654 |
| Expressed in insect cells | |||
| Burma | 72 ku | 1 | 660 |
| Pakistan | 63 ku | 112 | 660 |
| Burma | 62 ku | 112 | 636 |
| Pakistan | 55 ku | 112 | 607 |
| Pakistan | 53 ku | 112 | 578 |
| Burma | 50 ku | 112 | 534 |
| DNA vaccine | |||
| Burma | pJHEV | 1 | 660 |
| China | pSVL-ORF3 | 1 | 123 |
The genome sequence of HEV seems relatively stable[45]. The genome of strains isolated from geographically distinct locations is generally more diverse. At present, no consensus exists on genotype classification. The detected HEV strains are currently genetically characterized in laboratories on the basis of ORFs regions[32]. Recently, some research workers have also started to characterize HEV strains antigenetically using specific antibodies produced by the recombinant expressed capsid proteins[46,47]. On the basis of viruses having nucleotide divergence of not more than 20% of the nucleotides in the ORF2 region[48], the genomes of several HEV strains from different parts of the world can be grouped into at least four major genotypes[8,31,32,49]: Genotype 1 -including the isolates from South-East Asian (Burmese, some Indian strains), North and Central Asian (strains from China, Pakistan, Kyrgyzstan, and India), and North African strains; Genotype 2 -comprising the single North American (Mexico) isolate; Genotype 3 - consisting of the US and swine isolates; and Genotype 4 - including a subset of isolates from China and most isolates from Taiwan. Genetically heterogeneous isolates from several European countries have been designated new genotypes, but probably should be grouped with the US isolates into a large, heterogeneous group[31,50]. Two novel isolates of HEV have recently been described in Argentina. Distinct from all previously described isolates, they represent two diverse subtypes of a new genotype of HEV[51]. Despite the diversity of HEV genotype, no evidence has been found that heterogeneity results from the genetic diversity, thus HEV seems to exist as a single serotype[8-10].
At present, no commercially available vaccines exist for the prevention of hepatitis E. However, several studies for the development of an effective vaccine against hepatitis E are in progress[37,52-56]. Several lines of evidence have suggested the feasibility of a HEV vaccine. First, serum antibodies to HEV develop in response to naturally acquired and experimentally induced HEV infections in cynomolgus monkeys[57]. Second, seroepidemiology of hepatitis E suggests that people previously infected with HEV are protected during epidemics of the disease[58]. Finally, successful passive immune prophylaxis in animals indicated that effective vaccination against hepatitis E based on humoral immunity is possible[59]. Although different geographical isolates of HEV have been identified, only one serotype has been recognized[8]. So it may be possible to produce a broadly protective vaccine.
ORF2-encoded protein of HEV is the most promising subunit vaccine candidate because it possesses a good antigenicity. So far, HEV ORF2 gene or its fragments have been expressed in prokaryote cells[56,60-64], insect cells[37,65-68], yeast cells[69-71], animal cells[72], and plants (tomatoes)[30], etc., and the expression products possess immunogenicity.
It was reported recently that the smallest fragment of ORF2, which is capable of combining the neutralizing antibody of HEV, is located between 452 and 617 aa. This fragment does not only induce neutralizing antibody, but also cross react to the antibody of other genotype[22]. It was demonstrated that the 2/3 length of C-terminal region of HEV ORF2 contains epitopes, which are recognized by both acute-phase and convalescent-phase antibody to HEV and are likely to be associated with limited immunity to the infection, but these epitopes may be masked when larger portions of ORF2 are expressed as recombinant proteins[64]. So far, many different length of ORF2 fragments have been expressed in E. coli[56,63,64], but only a few expression products have been found with significant immunoactivity. The first candidate HEV vaccine was a recombinant fusion protein including 439 amino acids (221-660 aa). It comprised tryptophan synthetase and the carboxy terminal fragment of the ORF2 protein of the Burmese strain (genotype 1) and was expressed in E.coli. Two cynomolgus macaques were vaccinated with the fusion protein, and neither of them developed hepatitis following experimental challenge. The animals challenged with the heterologous HEV, the Mexican strain (genotype 2), did get infected, but did not develop hepatitis[73]. Another study also shows that the immunization with the bacterially expressed ORF2 peptide (pE2) corresponding to 394-607 aa, may prevent HEV infection in primates experimentally transmitted with the homologous strain of HEV[56].
It is thought that the best candidate at present for a hepatitis E vaccine is a recombinant HEV capsid antigen expressed in insect cells from a baculovirus vector[25]. When ORF2 of genotype 1 strain is expressed from a baculovirus vector in insect cells, the initial 72-ku protein is quickly processed to smaller proteins, possibly via a protease encoded by the baculovirus[66]. The most abundant proteins are 56 ku and 53 ku in size, respectively. However, the only neutralization epitope identified to date was mapped to a region of the ORF2 protein of Sar-55 between amino acids 578 and 607[74]. Therefore, the 56 ku protein (112-607 aa) but not the 53 ku protein (112-578 aa) should contain this epitope. The recombinant 56 ku protein that is more soluble than the full-length protein was an efficient immunogen when adjuvanted with alum[53,57]. Two 400 ng doses of the vaccine were injected to rhesus monkeys intramuscularly and high antibody titers (1:10000) were achieved. The monkeys were protected against hepatitis E following intravenous challenge with 300000 monkey infectious doses (MID50) of the homologous (Sar- 55) or 100 000 MID50 of a heterologous HEV (Mexican 14) [57] . To evaluate the immunogenicity and protective efficacy of the 53 kDa protein, the same research group immunized rhesus monkeys with the 53 ku vaccine, which was derived from processing of the ORF2 protein of Sar-55 and purified from the medium of recombinant baculovirus-infected insect cells and precipitated with alum. Two doses of vaccine containing 385 ng of alum-precipitated 53 ku protein were inoculated into the monkeys intramuscularly. The immunized monkeys were challenged with a high (1000 MID50) or low (100 MID 50) dose of homologous virus. The result showed vaccination with the 53 ku protein greatly reduced virus shedding, but did not protect against hepatitis following the high dose challenge. Virus was not detected in the vaccinated animals following the low dose challenge, suggesting that sterilizing immunity might have been achieved. This study indicated that the 53 ku protein did not function as a better vaccine than did the 56 ku protein and actually appeared to have been less effective in preventing disease[37]. The researches have shown that almost complete vaccine-induced protection lasts for at least 6 mo and partial protection persists for at least 1 year following vaccination[75].
Recently, yeast expression system has been successfully used for production of vaccines, for example, the recombinant hepatitis B surface antigen[76,77]. There are some advantages of using this expression system, such as the expression products similar to the natural protein, maintaining the biological activity of the production, to produce easily in large scale and so on[78]. In recent studies, the ORF2 of HEV (69-660 aa and 112-660 aa) was successfully expressed in pichia pastoris and the expression products of recombinant protein (59 ku) was purified and immunized to rhesus monkeys. High titer of anti-HEV (1:8000) was detected in the immunized monkeys[69-71,79]. Research on using plants for expression and delivery of oral vaccine has attracted much academic attention and has become a hot spot of study since 1990 when Curtiss et al[80] first reported the expression of Streptococcus mutants surface protein antigen A (SpaA) in tobacco, and great progress has been made since then[81]. In a recent study, the ORF2 partial gene of HEV named E2 (810 bp, 349-604 aa) was constructed into plasmid pCAMBIA1301 and yielded the reconstructed plant binary expression plasmid p1301E2[62]. The p1301E2 was expressed in tomatoes and the recombinant antigen derived from them has normal immunoactivity. The transgenic tomatoes may hold a good promise for producing a new type of low-cost oral vaccine for hepatitis E[30].
DNA immunization usually induces both cellular and antibody immune response. So it might provide a longer duration of protection. Therefore, it has become another focus of HEV vaccine research. Recombinant DNA vaccine is a recently developed new type of vaccine. Through directly injecting the recombinant plasmid DNA with the target gene into human or animals, the DNA will express the expected protein inside the host cells and therefore induce the immune responses to prevent and fight the disease. Recently, a new technology has been introduced for the development of subunit vaccines involving the direct injection of purified plasmid DNA containing protein coding sequences of interest and appropriate regulatory elements allowing expression in mammalian tissues. This novel technology has several potential advantages over other vaccine approaches[82]. First, the antigens expressed in living cells are in their native form, improving processing and presentation and usually resulting in the activation of both arms of the immune system. Second, DNA can be made inexpensively, in large quantities, at high levels of purity, and is extremely stable. Third, the vector is unlikely to be, or to become, pathogenic, in contrast to live-virus vaccines, and there is little or no immune response to the vector.
In an early study, an HEV cDNA pSVL-ORF3 was constructed by inserting the full length of ORF3 fragment into prokaryotic expression vector pSVL. A total amount of 100 μg of the cDNA was injected to BALB/c mice intramuscularly and anti-HEV IgG was detected in 12 of the 16 immunized mice. However, no antibody was found in the mice injected with the empty vector. The result indicated that the recombinant HEV cDNA could induce the antibody response in mice[83]. Later, another HEV cDNA pJHEV was constructed by inserting full length of ORF2 fragment. The HEV structural protein was expressed in Cos-7 cells under the control of a hCMV promoter. The successful construct was further tested in BALB/c mice for the induction of an ORF2 specific immune response. All the mice immunized with the cDNA were found seroconverted, but no anti- HEV responses induced in the mice of control group. Sera from the mice injected with pJHEV specifically recognized HEV ORF2 structural protein expressed in recombinant baculovirus in an enzyme-linked- immunosorbent assay (ELISA) and Western blot[82]. Furthermore, it was also shown that the antiserum generated by the DNA vaccine could bind specifically to native HEV[84]. Recently, a full-length HEV cDNA clone was constructed in a pSGI vector. The three ORFs were amplified separately and then reconstructed to the full-length clone. The in vitro transcribed RNA of the full-length cDNA clone was infective in a HepG2 tissue culture. Viral replication was detected for six passages with strand-specific PCR[85].
In spite of the above vaccine candidates, recently the recombinant hepatitis E virus (rHEV) virus -like particles (VLPs) are also the focuses of vaccine research for hepatitis E. With 111 amino acids truncated at the N-terminal, when the capsid protein of HEV was expressed in the baculovirus expression system, it was spontaneously assembled into virus-like particles[54]. Electron cryomicroscopy shows that these VLPs are formed with 60 copies of a 54 ku protein arranged in T = 1 symmetry[54,86,87]. As a mucosal immunogen, the VLPs have several advantages: they are composed of a single protein assembled into particles without nucleic acid, which makes them unable to replicate; they are easy to prepare and purify in large quantities, with a yield of approximately 1 mg /107 insect cells; rHEV VLPs are antigenically similar to the native virions; they are highly immunogenic in experimental animals when injected parenterally; they are very stable at low pH such as in stomach; and oral delivery of rHEV VLPs could induce the same immune responses as occur in natural infection[86,54].
In a previous study[87], mice were orally inoculated with purified rHEV VLPs without adjuvant. Serum IgM response was obtained within 2 wk after the first administration. Serum IgG and IgA were detected by 4 wk, and the intestinal IgA response was found at 8 wk post-immunization. Therefore, the oral immunization of rHEV VLPs is capable of inducing both systemic and intestinal antibody responses. However, since mice are not susceptible to HEV, the same group recently immunized cynomolgus monkeys orally with 10 mg of purified rHEV VLPs, serum IgM, IgG, and IgA responses were observed. All these antibody responses were obtained without adjuvants. When the monkeys were challenged with native HEV by intravenous injection, they were protected against infection or developing hepatitis. These results suggested that rHEV VLPs could be a candidate for the oral hepatitis E vaccine[88]. Some similar experiments have been described previously[89-91].
So many vaccine candidates for hepatitis E have been explored as described above. But so far, there is only one HEV vaccine candidate progressed to the stage of clinical trials. That is the 56 ku (expressed in insect cells from a baculovirus vector) recombinant vaccine developed at the NIH, USA[68]. In phase I trial, the vaccine was found to be safe and immunogenic in 88 American volunteers. A further phase I evaluation was performed in Nepal, where hepatitis E is endemic. Three doses of 5 μg and 20 μg, respectively, were injected into 22 Nepalese volunteers each at zero, one and six months. No serious adverse events were observed. By the second month, 43 of 44 volunteers had seroconverted to anti-HEV. By the 7th month, the remaining volunteers also developed antibody to HEV. The study indicated that the HEV vaccine candidate was safe and immunogenic. This same lot of vaccine is currently being used in phase II/III clinical trials in Nepal[25], where as many as 90% of the jaundice cases are caused by HEV.
In recent pre-clinical trails, the vaccine used in the experiment was from the same lot that was prepared for the above clinical trails. The results indicated that two doses of HEV vaccine as small as 1 μg were highly effective in preventing not only hepatitis but also infection following intravenous administration of 104 MID50 of virulent virus. However, these doses of vaccine will not be sufficient for long-term protection. Other studies have shown that a third dose of vaccine at 6 or 12 mo following the first dose will enhance immunogenicity and/or efficacy[75,92]. The study has also confirmed that the cross-protection against a Mexican genotype 2 isolate and extended the evidence for cross -protection to a US genotype 3 isolate of HEV. The results of this experiment have shown that the manufacture of a candidate hepatitis E vaccine can be scaled up to produce more clinical quality and that such a vaccine is highly immunogenic and effective for preventing hepatitis E in the pre -clinical trails. It is likely that protection against infection will be more effective following natural oral challenge with relatively small doses of HEV[25].
Despite the achievements mentioned above, there are still many questions to be answered in future. For example, there is a study showing that acute hepatitis E can be induced by plasma transfusion from a donor with HEV viremia, which indicates the possibility of transfusion transmitted hepatitis E[93]. Some similar studies also showed the possibility of post-transfusion hepatitis E[94-97]. Therefore, effective measures for preventing post-transfusion hepatitis E must be taken. To achieve effective immunization, how should the HEV vaccine be given by, orally or intramuscularly Will a monovalent vaccine protect against all HEV strains including HEV from animals and genotypes and provide a long-term immunity to hepatitis E To facilitate vaccine development and to improve our knowledge about the mechanism of virus replication, an effective practical cell culture system should be established and demonstrated.
| 1. | Harrison TJ. Hepatitis E virus -- an update. Liver. 1999;19:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 82] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Aggarwal R, Krawczynski K. Hepatitis E: an overview and recent advances in clinical and laboratory research. J Gastroenterol Hepatol. 2000;15:9-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 159] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Pérez-Gracia MT, Rodríguez-Iglesias M. [Hepatitis E virus: current status]. Med Clin (Barc). 2003;121:787-792. [PubMed] |
| 4. | Pringle CR. Virus taxonomy--San Diego 1998. Arch Virol. 1998;143:1449-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 140] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Pringle CR. Virus taxonomy--1999. The universal system of virus taxonomy, updated to include the new proposals ratified by the International Committee on Taxonomy of Viruses during 1998. Arch Virol. 1999;144:421-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 131] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Green KY, Ando T, Balayan MS, Berke T, Clarke IN, Estes MK, Matson DO, Nakata S, Neill JD, Studdert MJ. Taxonomy of the caliciviruses. J Infect Dis. 2000;181 Suppl 2:S322-S330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 341] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 7. | Berke T, Matson DO. Reclassification of the Caliciviridae into distinct genera and exclusion of hepatitis E virus from the family on the basis of comparative phylogenetic analysis. Arch Virol. 2000;145:1421-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Schlauder GG, Mushahwar IK. Genetic heterogeneity of hepatitis E virus. J Med Virol. 2001;65:282-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 238] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Meng XJ, Purcell RH, Halbur PG, Lehman JR, Webb DM, Tsareva TS, Haynes JS, Thacker BJ, Emerson SU. A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci USA. 1997;94:9860-9865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 862] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 10. | Wang Y, Zhang H, Ling R, Li H, Harrison TJ. The complete sequence of hepatitis E virus genotype 4 reveals an alternative strategy for translation of open reading frames 2 and 3. J Gen Virol. 2000;81:1675-1686. [PubMed] |
| 11. | VISWANATHAN R. A review of the literature on the epidemiology of infectious hepatitis. Indian J Med Res. 1957;45:145-155. [PubMed] |
| 12. | Khuroo MS. Study of an epidemic of non-A, non-B hepatitis. Possibility of another human hepatitis virus distinct from post-transfusion non-A, non-B type. Am J Med. 1980;68:818-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 429] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | Arora NK, Panda SK, Nanda SK, Ansari IH, Joshi S, Dixit R, Bathla R. Hepatitis E infection in children: study of an outbreak. J Gastroenterol Hepatol. 1999;14:572-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Naik SR, Aggarwal R, Salunke PN, Mehrotra NN. A large waterborne viral hepatitis E epidemic in Kanpur, India. Bull World Health Organ. 1992;70:597-604. [PubMed] |
| 15. | Velázquez O, Stetler HC, Avila C, Ornelas G, Alvarez C, Hadler SC, Bradley DW, Sepúlveda J. Epidemic transmission of enterically transmitted non-A, non-B hepatitis in Mexico, 1986-1987. JAMA. 1990;263:3281-3285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 102] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Schlauder GG, Dawson GJ, Erker JC, Kwo PY, Knigge MF, Smalley DL, Rosenblatt JE, Desai SM, Mushahwar IK. The sequence and phylogenetic analysis of a novel hepatitis E virus isolated from a patient with acute hepatitis reported in the United States. J Gen Virol. 1998;79:447-456. [PubMed] |
| 17. | Erker JC, Desai SM, Schlauder GG, Dawson GJ, Mushahwar IK. A hepatitis E virus variant from the United States: molecular characterization and transmission in cynomolgus macaques. J Gen Virol. 1999;80:681-690. [PubMed] |
| 18. | Takahashi K, Iwata K, Watanabe N, Hatahara T, Ohta Y, Baba K, Mishiro S. Full-genome nucleotide sequence of a hepatitis E virus strain that may be indigenous to Japan. Virology. 2001;287:9-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 110] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Zhuang H, Cao XY, Liu CB, Wang GM. Epidemiology of hepatitis E in China. Gastroenterol Jpn. 1991;26 Suppl 3:135-138. [PubMed] |
| 20. | Zhuang H, Zhu WF, Li F, Zhu XJ, Li K, Cui YH, Zhu YH. Studies on hepatitis E. Chin Med Sci J. 1999;14:S47-50. |
| 21. | Skidmore S. Overview of Hepatitis E Virus. Curr Infect Dis Rep. 2002;4:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Meng J, Dai X, Chang JC, Lopareva E, Pillot J, Fields HA, Khudyakov YE. Identification and characterization of the neutralization epitope(s) of the hepatitis E virus. Virology. 2001;288:203-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 132] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Schofield DJ, Purcell RH, Nguyen HT, Emerson SU. Monoclonal antibodies that neutralize HEV recognize an antigenic site at the carboxyterminus of an ORF2 protein vaccine. Vaccine. 2003;22:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Meng J, Dubreuil P, Pillot J. A new PCR-based seroneutralization assay in cell culture for diagnosis of hepatitis E. J Clin Microbiol. 1997;35:1373-1377. [PubMed] |
| 25. | Purcell RH, Nguyen H, Shapiro M, Engle RE, Govindarajan S, Blackwelder WC, Wong DC, Prieels JP, Emerson SU. Pre-clinical immunogenicity and efficacy trial of a recombinant hepatitis E vaccine. Vaccine. 2003;21:2607-2615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 100] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Zhang J, Ge SX, Huang GY, Li SW, He ZQ, Wang YB, Zheng YJ, Gu Y, Ng MH, Xia NS. Evaluation of antibody-based and nucleic acid-based assays for diagnosis of hepatitis E virus infection in a rhesus monkey model. J Med Virol. 2003;71:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Grimm AC, Fout GS. Development of a molecular method to identify hepatitis E virus in water. J Virol Methods. 2002;101:175-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Tam AW, White R, Yarbough PO, Murphy BJ, McAtee CP, Lanford RE, Fuerst TR. In vitro infection and replication of hepatitis E virus in primary cynomolgus macaque hepatocytes. Virology. 1997;238:94-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Huang R, Li D, Wei S, Li Q, Yuan X, Geng L, Li X, Liu M. Cell culture of sporadic hepatitis E virus in China. Clin Diagn Lab Immunol. 1999;6:729-733. [PubMed] |
| 30. | Ma Y, Lin SQ, Gao Y, Li M, Luo WX, Zhang J, Xia NS. Expression of ORF2 partial gene of hepatitis E virus in tomatoes and immunoactivity of expression products. World J Gastroenterol. 2003;9:2211-2215. [PubMed] |
| 31. | Hepatitis E. World health organization. WHO/CDS/CSR/EDC/. 2001;12. |
| 32. | Worm HC, van der Poel WH, Brandstätter G. Hepatitis E: an overview. Microbes Infect. 2002;4:657-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 108] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Emerson SU, Purcell RH. Recombinant vaccines for hepatitis E. Trends Mol Med. 2001;7:462-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Magden J, Takeda N, Li T, Auvinen P, Ahola T, Miyamura T, Merits A, Kääriäinen L. Virus-specific mRNA capping enzyme encoded by hepatitis E virus. J Virol. 2001;75:6249-6255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 129] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 35. | Kabrane-Lazizi Y, Meng XJ, Purcell RH, Emerson SU. Evidence that the genomic RNA of hepatitis E virus is capped. J Virol. 1999;73:8848-8850. [PubMed] |
| 36. | Zafrullah M, Ozdener MH, Panda SK, Jameel S. The ORF3 protein of hepatitis E virus is a phosphoprotein that associates with the cytoskeleton. J Virol. 1997;71:9045-9053. [PubMed] |
| 37. | Zhang M, Emerson SU, Nguyen H, Engle RE, Govindarajan S, Gerin JL, Purcell RH. Immunogenicity and protective efficacy of a vaccine prepared from 53 kDa truncated hepatitis E virus capsid protein expressed in insect cells. Vaccine. 2001;20:853-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Zafrullah M, Ozdener MH, Kumar R, Panda SK, Jameel S. Mutational analysis of glycosylation, membrane translocation, and cell surface expression of the hepatitis E virus ORF2 protein. J Virol. 1999;73:4074-4082. [PubMed] |
| 39. | Tam AW, Smith MM, Guerra ME, Huang CC, Bradley DW, Fry KE, Reyes GR. Hepatitis E virus (HEV): molecular cloning and sequencing of the full-length viral genome. Virology. 1991;185:120-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 837] [Cited by in RCA: 816] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 40. | Zhang Y, McAtee P, Yarbough PO, Tam AW, Fuerst T. Expression, characterization, and immunoreactivities of a soluble hepatitis E virus putative capsid protein species expressed in insect cells. Clin Diagn Lab Immunol. 1997;4:423-428. [PubMed] |
| 41. | Ghabrah TM, Tsarev S, Yarbough PO, Emerson SU, Strickland GT, Purcell RH. Comparison of tests for antibody to hepatitis E virus. J Med Virol. 1998;55:134-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 42. | Anderson DA, Li F, Riddell M, Howard T, Seow HF, Torresi J, Perry G, Sumarsidi D, Shrestha SM, Shrestha IL. ELISA for IgG-class antibody to hepatitis E virus based on a highly conserved, conformational epitope expressed in Escherichia coli. J Virol Methods. 1999;81:131-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Li TC, Zhang J, Shinzawa H, Ishibashi M, Sata M, Mast EE, Kim K, Miyamura T, Takeda N. Empty virus-like particle-based enzyme-linked immunosorbent assay for antibodies to hepatitis E virus. J Med Virol. 2000;62:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 44. | Obriadina A, Meng JH, Ulanova T, Trinta K, Burkov A, Fields HA, Khudyakov YE. A new enzyme immunoassay for the detection of antibody to hepatitis E virus. J Gastroenterol Hepatol. 2002;17 Suppl 3:S360-S364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Arankalle VA, Paranjape S, Emerson SU, Purcell RH, Walimbe AM. Phylogenetic analysis of hepatitis E virus isolates from India (1976-1993). J Gen Virol. 1999;80:1691-1700. [PubMed] |
| 46. | Riddell MA, Li F, Anderson DA. Identification of immunodominant and conformational epitopes in the capsid protein of hepatitis E virus by using monoclonal antibodies. J Virol. 2000;74:8011-8017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Wang Y, Zhang H, Li Z, Gu W, Lan H, Hao W, Ling R, Li H, Harrison TJ. Detection of sporadic cases of hepatitis E virus (HEV) infection in China using immunoassays based on recombinant open reading frame 2 and 3 polypeptides from HEV genotype 4. J Clin Microbiol. 2001;39:4370-4379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 48. | Ando T, Noel JS, Fankhauser RL. Genetic classification of "Norwalk-like viruses. J Infect Dis. 2000;181 Suppl 2:S336-S348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 220] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 49. | Wang Y, Ling R, Erker JC, Zhang H, Li H, Desai S, Mushahwar IK, Harrison TJ. A divergent genotype of hepatitis E virus in Chinese patients with acute hepatitis. J Gen Virol. 1999;80:169-177. [PubMed] |
| 50. | Schlauder GG, Desai SM, Zanetti AR, Tassopoulos NC, Mushahwar IK. Novel hepatitis E virus (HEV) isolates from Europe: evidence for additional genotypes of HEV. J Med Virol. 1999;57:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 51. | Schlauder GG, Frider B, Sookoian S, Castaño GC, Mushahwar IK. Identification of 2 novel isolates of hepatitis E virus in Argentina. J Infect Dis. 2000;182:294-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 52. | Purcell RH, Emerson SU. Hepatitis E virus. In Knipe DM, Howley PM, Fields Virology, Fourth edition, V2 Lippincot and Wilkins. Philadelphia, PA. 2001;3051-3052. |
| 53. | Tsarev SA, Tsareva TS, Emerson SU, Govindarajan S, Shapiro M, Gerin JL, Purcell RH. Recombinant vaccine against hepatitis E: dose response and protection against heterologous challenge. Vaccine. 1997;15:1834-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 96] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 54. | Xing L, Kato K, Li T, Takeda N, Miyamura T, Hammar L, Cheng RH. Recombinant hepatitis E capsid protein self-assembles into a dual-domain T = 1 particle presenting native virus epitopes. Virology. 1999;265:35-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 106] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 55. | Yarbough PO. Hepatitis E virus. Advances in HEV biology and HEV vaccine approaches. Intervirology. 1999;42:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 56. | Im SW, Zhang JZ, Zhuang H, Che XY, Zhu WF, Xu GM, Li K, Xia NS, Ng MH. A bacterially expressed peptide prevents experimental infection of primates by the hepatitis E virus. Vaccine. 2001;19:3726-3732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 57. | Tsarev SA, Tsareva TS, Emerson SU, Govindarajan S, Shapiro M, Gerin JL, Purcell RH. Successful passive and active immunization of cynomolgus monkeys against hepatitis E. Proc Natl Acad Sci USA. 1994;91:10198-10202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 154] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 58. | Bryan JP, Tsarev SA, Iqbal M, Ticehurst J, Emerson S, Ahmed A, Duncan J, Rafiqui AR, Malik IA, Purcell RH. Epidemic hepatitis E in Pakistan: patterns of serologic response and evidence that antibody to hepatitis E virus protects against disease. J Infect Dis. 1994;170:517-521. [RCA] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 114] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 59. | Quiroga JA, Cotonat T, Castillo I, Carreño V. Hepatitis E virus seroprevalence in acute viral hepatitis in a developed country confirmed by a supplemental assay. J Med Virol. 1996;50:16-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 60. | Zhang M, Zhao H, Jiang Y. [Expression of hepatitis E virus structural gene in E. coli]. Zhonghua Shiyan He Linchuang Bingduxue Zazhi. 1999;13:130-132. [PubMed] |
| 61. | Bi S, Lu J, Jiang L, Huang G, Pan H, Jiang Y, Zhang M, Shen X. [Preliminary evidence that a hepatitis E virus (HEV) ORF2 recombinant protein protects cynomolgus macaques against challenge with wild-type HEV]. Zhonghua Shiyan He Linchuang Bingduxue Zazhi. 2002;16:31-32. [PubMed] |
| 62. | Li SW, Zhang J, He ZQ, Ge SX, Gu Y, Lin J, Liu RS, Xia NS. [The study of aggregate of the ORF2 peptide of hepatitis E virus expressed in Escherichia coli]. Shengwu Gongchengxue Bao. 2002;18:463-467. [PubMed] |
| 63. | Li F, Torresi J, Locarnini SA, Zhuang H, Zhu W, Guo X, Anderson DA. Amino-terminal epitopes are exposed when full-length open reading frame 2 of hepatitis E virus is expressed in Escherichia coli, but carboxy-terminal epitopes are masked. J Med Virol. 1997;52:289-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 64. | Li F, Riddell MA, Seow HF, Takeda N, Miyamura T, Anderson DA. Recombinant subunit ORF2.1 antigen and induction of antibody against immunodominant epitopes in the hepatitis E virus capsid protein. J Med Virol. 2000;60:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 65. | Zhang M, Yi Y, Zhan M, Liu C, Bi S. [Expression of thermal stable, soluble hepatitis E virus recombinant antigen]. Zhonghua Shiyan He Linchuang Bingduxue Zazhi. 2002;16:20-22. [PubMed] |
| 66. | Robinson RA, Burgess WH, Emerson SU, Leibowitz RS, Sosnovtseva SA, Tsarev S, Purcell RH. Structural characterization of recombinant hepatitis E virus ORF2 proteins in baculovirus-infected insect cells. Protein Expr Purif. 1998;12:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 116] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 67. | McAtee CP, Zhang Y, Yarbough PO, Fuerst TR, Stone KL, Samander S, Williams KR. Purification and characterization of a recombinant hepatitis E protein vaccine candidate by liquid chromatography-mass spectrometry. J Chromatogr B Biomed Appl. 1996;685:91-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 68. | Stevenson P. Nepal calls the shots in hepatitis E virus vaccine trial. Lancet. 2000;355:1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 69. | Tong YP, Bi SL, Jiang YZ, Zhan MY. Intracellular expression of hepatitis E virus ORF2 protein in pichia pastoris and its purification. Bingdu Xuebao. 2001;17:34-37. |
| 70. | Tong YP, Bi SL, Zhang MC, Lu J, Zhan MY. Extracellular expres-sion of hepatitis E virus ORF2 protein in pichia pastoris. Zhonghua Shiyan He Linchuangbing Duxue Zazhi. 2000;14:391-392. |
| 71. | Tong YP, Lu J, Jian YZ, Bi SL, Zhan MY. The application of re-combinant HEV ORF2 protein expressed in yeast cells to the di- agnosis of hepatitis E. Zhonghua Shiyan He Linchuang Bingduxue Zazhi. 2001;15:189-190. |
| 72. | Jameel S, Zafrullah M, Ozdener MH, Panda SK. Expression in animal cells and characterization of the hepatitis E virus structural proteins. J Virol. 1996;70:207-216. [PubMed] |
| 73. | Purdy MA, McCaustland KA, Krawczynski K, Spelbring J, Reyes GR, Bradley DW. Preliminary evidence that a trpE-HEV fusion protein protects cynomolgus macaques against challenge with wild-type hepatitis E virus (HEV). J Med Virol. 1993;41:90-94. [RCA] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 81] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 74. | Schofield DJ, Glamann J, Emerson SU, Purcell RH. Identification by phage display and characterization of two neutralizing chimpanzee monoclonal antibodies to the hepatitis E virus capsid protein. J Virol. 2000;74:5548-5555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 130] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 75. | Zhang M, Emerson SU, Nguyen H, Engle R, Govindarajan S, Blackwelder WC, Gerin J, Purcell RH. Recombinant vaccine against hepatitis E: duration of protective immunity in rhesus macaques. Vaccine. 2002;20:3285-3291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 76. | Torresi J, Earnest-Silveira L, Deliyannis G, Edgtton K, Zhuang H, Locarnini SA, Fyfe J, Sozzi T, Jackson DC. Reduced antigenicity of the hepatitis B virus HBsAg protein arising as a consequence of sequence changes in the overlapping polymerase gene that are selected by lamivudine therapy. Virology. 2002;293:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 170] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 77. | Zhang YX, Dai L, Sun XM. Kinetic aspect of hepatitis B surface antigen production in recombinant Saccharomyces cerevisiae fermentation. Process Biochemistry. 2003;38:1593-1598. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 78. | Romanos M. Advances in the use of pichia pastoris for high-level gene expression. Curr Opin Biotechnol. 1995;6:527-533. [RCA] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 141] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 79. | Lu J, Tong YP, Jiang YZ, Bi SL. The application of recombinant HEV ORF2 protein expressed in yeast cells to the detection of serum antibody in experimental rhesus monkeys. Zhonghua Shiyan He Linchuang Bingduxue Zazhi. 2001;15:382-383. |
| 80. | Curtiss R, Galan JE, Nakayama K, Kelly SM. Stabilization of recombinant avirulent vaccine strains in vivo. Res Microbiol. 1990;141:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 81. | Gao Y, Ma Y, Li M, Cheng T, Li SW, Zhang J, Xia NS. Oral immunization of animals with transgenic cherry tomatillo expressing HBsAg. World J Gastroenterol. 2003;9:996-1002. [PubMed] |
| 82. | He J, Hoffman SL, Hayes CG. DNA inoculation with a plasmid vector carrying the hepatitis E virus structural protein gene induces immune response in mice. Vaccine. 1997;15:357-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 83. | Lu F, Zhuang H, Zhu Y, Zhu X. A preliminary study on immune response to hepatitis E virus DNA vaccine in mice. Chin Med J (Engl). 1996;109:919-921. [PubMed] |
| 84. | He J, Binn LN, Caudill JD, Asher LV, Longer CF, Innis BL. Antiserum generated by DNA vaccine binds to hepatitis E virus (HEV) as determined by PCR and immune electron microscopy (IEM): application for HEV detection by affinity-capture RT-PCR. Virus Res. 1999;62:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 85. | Panda SK, Ansari IH, Durgapal H, Agrawal S, Jameel S. The in vitro-synthesized RNA from a cDNA clone of hepatitis E virus is infectious. J Virol. 2000;74:2430-2437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 116] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 86. | Li TC, Yamakawa Y, Suzuki K, Tatsumi M, Razak MA, Uchida T, Takeda N, Miyamura T. Expression and self-assembly of empty virus-like particles of hepatitis E virus. J Virol. 1997;71:7207-7213. [PubMed] |
| 87. | Li T, Takeda N, Miyamura T. Oral administration of hepatitis E virus-like particles induces a systemic and mucosal immune response in mice. Vaccine. 2001;19:3476-3484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 88. | Li TC, Suzaki Y, Ami Y, Dhole TN, Miyamura T, Takeda N. Protection of cynomolgus monkeys against HEV infection by oral administration of recombinant hepatitis E virus-like particles. Vaccine. 2004;22:370-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 89. | Rose RC, Lane C, Wilson S, Suzich JA, Rybicki E, Williamson AL. Oral vaccination of mice with human papillomavirus virus-like particles induces systemic virus-neutralizing antibodies. Vaccine. 1999;17:2129-2135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 90. | Ball JM, Hardy ME, Atmar RL, Conner ME, Estes MK. Oral immunization with recombinant Norwalk virus-like particles induces a systemic and mucosal immune response in mice. J Virol. 1998;72:1345-1353. [PubMed] |
| 91. | Estes MK, Ball JM, Guerrero RA, Opekun AR, Gilger MA, Pacheco SS, Graham DY. Norwalk virus vaccines: challenges and progress. J Infect Dis. 2000;181 Suppl 2:S367-S373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 92. | Safary A. Perspectives of vaccination against hepatitis E. Intervirology. 2001;44:162-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 93. | Xia NS, Zhang J, Zheng YJ, Qiu Y, Ge SX, Ye XZ, Ou SH. [Detection of hepatitis E virus on a blood donor and its infectivity to rhesus monkey]. Zhonghua Ganzangbing Zazhi. 2004;12:13-15. [PubMed] |
| 94. | Nicand E, Grandadam M, Teyssou R, Rey JL, Buisson Y. Viraemia and faecal shedding of HEV in symptom-free carriers. Lancet. 2001;357:68-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 95. | Arankalle VA, Chobe LP. Retrospective analysis of blood transfusion recipients: evidence for post-transfusion hepatitis E. Vox Sang. 2000;79:72-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 84] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 96. | Arankalle VA, Chobe LP. Hepatitis E virus: can it be transmitted parenterally. J Viral Hepat. 1999;6:161-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 97. | Gao DY, Peng G, Zhu JM, Sun L, Zheng YJ, Zhang J. [Investigation of sub-clinical infection of hepatitis E virus in blood donors]. Zhonghua Ganzangbing Zazhi. 2004;12:11-12. [PubMed] |
Edited by Kumar M Proofread by Xu FM