Published online Jul 15, 2004. doi: 10.3748/wjg.v10.i14.2119
Revised: January 2, 2004
Accepted: January 15, 2004
Published online: July 15, 2004
AIM: To induce efficient expansion of natural killer (NK) cells from peripheral blood mononuclear cells (PBMCs) using a culture of anchorage-dependent Wilms tumor cell lines, and to provide a reliable supply for adoptive immunotherapy of hepatocellular carcinoma.
METHODS: Culture expansion of NK cells was achieved using PBMCs cultured with Wilms tumor cells. Cytotoxicity was measured using a standard 51Cr release assay and crystal violet staining technique. The proportions of CD3+, CD4+, CD8+, CD16+, and CD56+ cells were determined by flow cytometry.
RESULTS: After PBMCs from healthy donors and hepatocellular carcinoma (HCC) were cultured with irradiated HFWT cells for 10-21 d, CD56+CD16+ cells shared more than 50% of the cell population, and more than 80% of fresh HFWT cells were killed at an effector/target ratio of 2 over 24 h. NK-enriched lymphocyte population from HCC patients killed HCC-1 and 2 cells with sensitivities comparable to fresh TKB-17RGB cells. HCC cells proliferated 196-fold with the irradiated HFWT cells at 18 d. Stimulation by HFWT cells required intimate cell-cell interaction with PBMC. However, neither the soluble factors released from HFWT cells nor the fixed HFWT cells were effective for NK expansion. The lymphocytes expanded with IL-2 killed fresh HFWT target cells more effectively than the lymphocytes expanded with the 4-cytokine cocktail (IL-l β, IL-2, IL-4 and IL-6). IL-2 was the sole cytokine required for NK expansion.
CONCLUSION: Wilms tumor is sensitive to human NK cells and is highly efficient for selective expansion of NK cells from PBMCs.
- Citation: Peng BG, Liang LJ, He Q, Huang JF, Lu MD. Expansion and activation of natural killer cells from PBMC for immunotherapy of hepatocellular carcinoma. World J Gastroenterol 2004; 10(14): 2119-2123
- URL: https://www.wjgnet.com/1007-9327/full/v10/i14/2119.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i14.2119
Natural killer (NK) cells are CD3-CD56+ and/or CD16+ cytotoxic lymphocytes that mediate first-line defense against various types of target cells without prior immunization[1,2]. The regulation mechanism of human natural killer (NK) cell growth has not been well characterized despite the importance of NK cells in immune response[3]. One reason is that the currently used culture system for human NK cells is relatively poor at inducing a strong growth response compared with culture systems for other lymphocytes, such as T cells.
Since K562 cells expressing scarcely MHC-class I on their surface are highly sensitive to natural killer (NK) cells, they have been widely used for the assay of NK killing activity[4-6]. When K562 cells are killed by NK cells in vitro, apparent growth response of NK cells follows. The stimulation by K562 requires direct cell-cell contact and is not reconstituted by cell-free supernatants. However, the stimulation is not necessarily sufficient for the NK selective expansion in peripheral blood mononuclear cells (PBMCs) of every subject. Reports from Perussia et al[7] and Silva et al[8,9] suggested that human B lymphoblastoid cell lines and leukapheresed peripheral blood stem cell grafts were also useful for human NK cell expansion. Sekine et al[10] developed an alternative method for lymphocyte expansion from peripheral blood by cultivating cells with IL-2 and immobilized anti-CD3 monoclonal antibodies. Application of expensive anti-CD3, anti-CD16 bispecific antibodies may avoid the dilution of NK cells in the lymphocyte populations[11]. Coculture of NK cells with dendritic cells (DCs) resulted in significant enhancement of NK cell cytotoxicity and IFN-gamma production[12]. Coexpression of GM-CSF and B70 may enhance NK-mediated cytotoxicity, and then induce the antitumor immunity in hepatoma transplanted into nude mice[13].We consider that, for further use of the NK cells in adoptive immunotherapy of human tumors, clear separation of expanded NK cells and suspension cultured allogeneic EB virus-transformed cells that may have escaped from the killing by NK cells will be difficult.
In this study, we screened anchorage-dependent virus-free human tumor cell lines as an appropriate target in the NK cell expansion culture. We found that an anchorage-dependent cell line derived from Wilms tumor (HFWT) was sensitive to human NK cells.
All the cell lines were from routine stock cultures in the RIKEN Cell Bank. Cell lines were maintained in basal medium containing 100 mL/L or 150 mL/L fetal bovine serum (FBS). Peripheral blood was taken from healthy volunteers and hepatocellular carcinomas (HCCs) were from patients who gave their informed consent. Recombinant human IL-1 β, -2, -4, -6, -7, -12, and -15 were purchased from Genzyme (Tokyo, Japan). Mouse anti-human-CD3, CD4, -CD8, CD56 and CD16 monoclonal antibodies were purchased from Nichirei Co., (Tokyo, Japan). 51Cr was purchased from Nen Life Science Products Inc. (Boston, USA).
Suspended cells (l × 106) were washed three times with PBS, incubated for 30 min with monoclonal antibodies, 30 min with FITC-labeled goat anti-mouse IgG polyclonal antibody. The cells were again washed with PBS containing 40 mL/L FBS. They were re-suspended in the same buffer at a concentration of l × 106/mL and were immediately analyzed by FACS (Becton-Dickinson, Co.). The proportion of CD3+, CD4+, CD8+, CD16+, and CD56+ cells was detected with corresponding monoclonal antibodies.
HFWT cells (1 × 105/mL, 1 mL) were plated in a 24-well plate and incubated overnight in a humidified 50 mL/L CO2 incubator. The culture medium was replaced with PBS, and the cells were fixed with 0.5 mL of 40 g/L formaldehyde or 3:1 methanol-acetic acid mixture for 1 h, and then thoroughly washed with water.
HFWT cells were subjected to heat treatment at 100 °C for 2 s in a microwave oven after the replacement of the culture medium with PBS. The treated HFWT cell concentration was adjusted to 1 × 105/well for the NK expansion experiments.
PBMCs were prepared from heparinized peripheral blood with the conventional preparation kit (Lymphopre, Nycomed Pharma A.S., Norway). The cells were washed once with PBS, then once with RHAM alpha medium supplemented with 50 mL/L autologous plasma, and centrifuged at 1400 r/min (240 g) for 10 min at room temperature. Before addition of PBMCs to the NK cell expansion cultures, the confluent target tumor cells maintained in a 24-well plate were irradiated with 50 Gy of X-rays. The PBMCs (l × 106/mL, approximately 1 mL/well) were then cultured with the tumor cells (at this stage, the responder/stimulator ratio was adjusted to 10 in the culture medium, i.e. the RHAMα medium was supplemented just before adding 50 mL/L autologous plasma and a 4-cytokine cocktail of IL-l β (167 U/mL), IL-2 (67 U/mL), IL-4 (67 U/mL), and IL-6 (134 U/mL)). When IL-2 alone was used, the concentration was adjusted to 200 U/mL. IL-7, IL-12, and IL-15 were used at a concentration of 10 U/mL, 10 ng/mL, and 20 U/mL, respectively.
NK expansion culture was continued with appropriate changes of the medium, including addition of the indicated cytokines (at least half of the medium was changed every 2 d), until the adherent target cells disappeared. When K562 cells were the targets, this period was set at 7 d. The cell suspension was diluted to 5 × 105/mL and the culture continued. Whenever the cell suspension reached 5 × 106/mL, the dilution was repeated.
A standard 51Cr release assay was performed in the 4-h co-culture of the effector lymphocytes and the target K562 cells as described[14]. The crystal violet staining was also used[15]. Briefly, the target cells, 1 × 104/well suspended in 200 μL RHAM α medium containing 50 mL/L plasma from the lymphocyte donor (or 50 mL/L PPF whenever the plasma was in short supply), were seeded in a 96-well plate and were pre-cultured overnight. The cultured target cells were washed once with PBS, then the cultured lymphocytes suspended in 200 μL of RHAM α medium containing 50 mL/L autologous plasma (or 50 mL/L PPF) were added as effector cells to each well at the indicated effector/target (E/T) ratio. The cells were co-cultured for 4 or 24 h. Then, the wells were washed once gently with appropriate amounts of Dulbecco’s PBS containing calcium and magnesium. The target cells remaining adhered were fixed for 1 h with 40 g/L formaldehyde (200 μL/well), and then stained with crystal violet solution (4 g/L in water, 100 μL/well) for 30 min at room temperature. The plate was washed with water and dried at room temperature. A 200 μL of 800 mL/L methanol was added into each well and the absorbance at 570 nm (A570) of each well was determined. As a 100% control, the A570 of the target cells cultured in a separate plate was determined just before the addition of the effector cells.
Percentage of surviving target cells was defined as follows: Surviving target cells (%) = (A - B)/(C - D) × 100%
Where A is the A570 of the well containing the target cells and the effector cells, B is the A570 of the well containing only the effector cells which remained in the well after the washing with calcium- and magnesium-containing Dulbecco’s PBS, C is the A570 of the 100% control target cells just before the addition of the effector cells, and D is the A570 of the well containing medium alone. The target cells cultured at an E/T ratio of 0 grew rapidly over the 24-h incubation period and, therefore, showed more than 100% survival.
Two hundred and forty kinds of human cell lines from RIKEN Cell Bank were screened for their expression of MHC-class I and class II surface molecules. Ten cell lines including leukemia cell line K562, HFWT (Wilms tumor), HMV-II (melanoma) and NB 19 (neuroblastoma) were found that weakly expressed MHC molecules of both.
Subsequently, PBMCs taken from healthy subjects were co-cultured with these cell lines after the target cells had been irradiated with 50 Gy of X-rays. HFWT cultures demonstrated a striking change in anchorage-dependent HFWT cells that were totally killed and disappeared after 13-14 d. CD3-CD56+ cells occupied 64.6%, 54.6%, and 75.9% of the lymphocyte population in the three experiments, respectively. However, K562 and HMV-II in experiments 2 and 3 only raised the proportion of CD3-CD56+ cells to 17.6% and 18.9%, respectively, though these proportions were higher than those (5.4%-13.0%) in the control cultures containing no target cells. In contrast, irradiated TKB-17RGB cells increased CD3+CD56 T cells in the lymphocyte populations in the three experiments to 95.1%, 96.0%, and 84.8%, respectively as compared to 74.4%, 58.2% and 60.9% in controls.
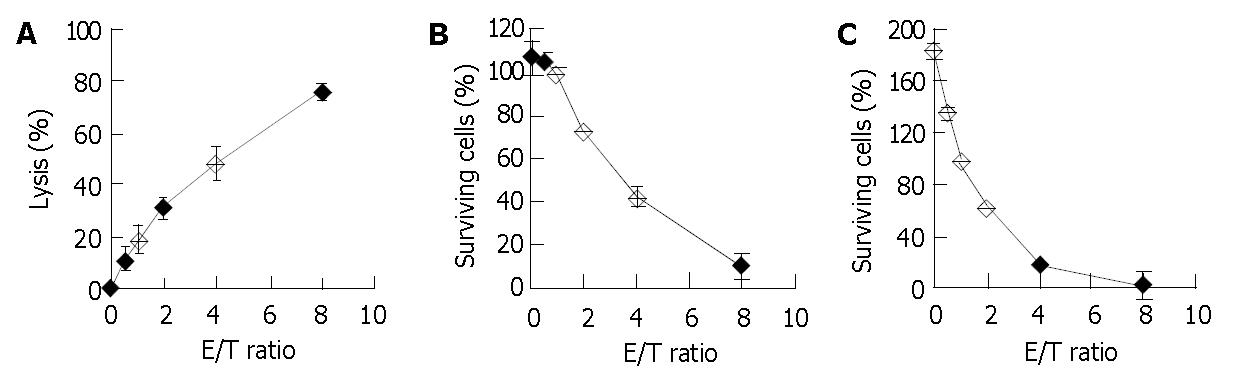
Figure 1A depicts a typical dose-response curve for the 4-h 51Cr release assay. The NK-enriched population lysed 31.3% and 76.5% of the fresh HFWT cells at E/T ratios of 2 and 8, respectively. A mirror image of this curve was observed in crystal violet staining (Figure 1B). The target cells showed 100% survival in crystal violet staining and 20% lysis in the 51Cr release assay when E/T ratio was 1.
Effect of extending co-culture time to 24 h was also examined. The percentage of the surviving control target cells usually exceeded 100% (Figure 1C) at an E/T ratio of 0, but the shoulder portion of the dose-response curve in Figure 1B disappeared and fewer surviving target cells were observed at larger E/T ratios. Only 17.0% of the target cells remained at E/T ratio of 4, whereas 42.5% remained in the 4-h crystal violet staining. Therefore, for determination of killing activity, 24-h crystal violet staining had a higher sensitivity than 4-h crystal violet staining at low E/T ratios.
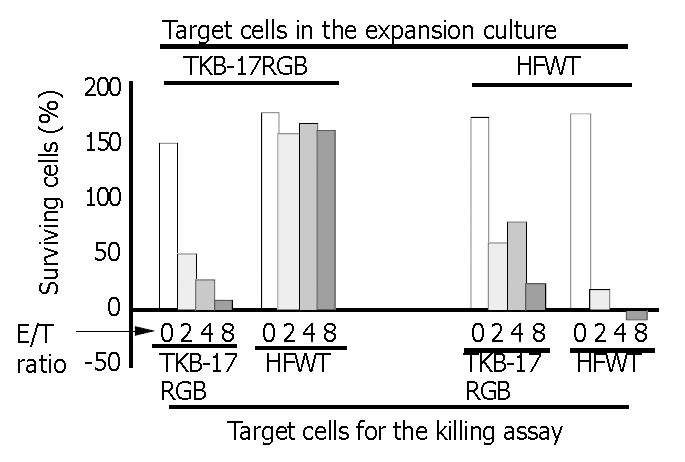
PBMCs grown on TKB-17RGB target cells could efficiently kill fresh TKB-17RGB cells but not fresh 17 RGB cells. Lymphocytes grown on the HFWT target cells could efficiently kill both fresh TKB-17RGB cells and fresh HFWT cells (Figure 2, 8 columns on the right), indicating that the lymphocytes contained nonspecific NK cells. The latter lymphocyte population, at an E/T ratio of 2 at 24 h, reduced TKB- 17R GB target cells to 60.5% and HFWT target cells to 18.5% compared to the control tumor cells (E/T ratio of 0) that proliferated to 175%-180% of starting levels.
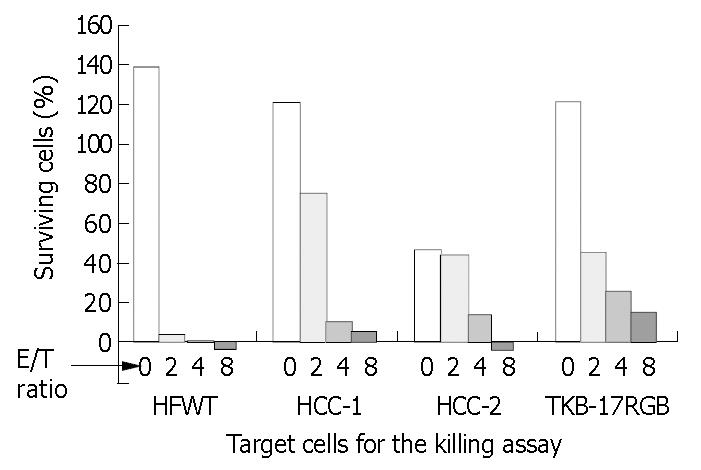
Lymphocytes from PBMC of HCC patient were cultured on the irradiated cells and proliferated 196-fold at 18 d. This was about 6 times the proliferation of lymphocytes suspended with the irradiated K562 cells (data not shown) and the proliferation ceased after 14 d in the NK expansion culture. X-ray irradiated HCC-1 cells (MHC class I positive) and HCC-2 cells (MHC class I negative) obtained from the same hepatocellular carcinoma were used in place of HFWT cells, and neither of the two cell lines could support efficient growth of the lymphocytes from PBMC of HCC patient. NK-enriched lymphocyte population from HCC patient, however, killed HCC-1 and 2 cells with sensitivity comparable to fresh TKB- 17RGB cells (Figure 3). About half of the control HCC-2 cells detached spontaneously after 24-h incubation. The NK-enriched population derived from the patient and expanded on the irradiated HFWT cells could also kill fresh K562 cells with high efficiency (data not shown).
| Irradiated target cells used for expansion culture | Cell proportion in lymphocyte populations (%) | |||
| CD3+CD56- | CD3-CD56+ | CD3+CD56+ | CD16+CD56+ | |
| HFWT | 15.2 | 72.0 | 12.3 | 72.7 |
| HCC-2 | 49.7 | 18.5 | 31.1 | 22.8 |
In a separate experiment, the effect on NK expansion of HFWT and MHC class I-non-expressing HCC-2 cells were compared using a medium containing IL-2 (Table 1). After 10 d of co-culture with PBMCs from Subject 1, irradiated HFWT cells induced 72.0% concentration of CD3-CD56+ cells, whereas irradiated HCC-2 cells induced only 18.5% concentration of CD3-CD56+ cells. CD16+CD56+ cells co-cultured with HFWT grew to 72.7% of the lymphocyte population, whereas CD16+CD56+ cells co-cultured with HCC-2 grew to 22.8% of the lymphocyte population. All the other tumor cell lines showed lower similar efficiency for NK cell expansion.
After PBMCs from Subject 1 were grown for 10 d in direct-contact co-culture, the final proportion of CD16+CD56+ cells reached 71.7%, but in the case of PBMCs separated from HFWT cells with a membrane filter, the final proportion reached only 3.9% (Table 2). Most of the lymphocytes in the latter culture were T lymphocytes. These results demonstrate that direct cell-cell interaction is crucial for NK expansion.
| PBMCs and HFWT co-culture conditions | Cell proportion in final lymphocyte populations (%) | ||
| CD3+CD56- | CD3-CD56+ | CD16+CD56+ | |
| Without separation | 13.4 | 78.2 | 71.7 |
| Separated by a membrane filter | 83.4 | 5.3 | 3.9 |
The highest increase in lymphocyte number from Subject-2 reached 401-fold after 15 d in the expansion culture. Lymphocytes derived from both Subject-2 and Subject-3 expanded with IL-2-containing medium killed fresh HFWT target cells more effectively than the lymphocytes expanded with the 4-cytokine cocktail (data not shown). Without the presence of target HFWT cells, lymphokine activated killer (LAK) cells grew slower than those cultured on the target cells. Unlike IL-2, IL-12 inhibited and IL-15 stimulated the growth of lymphocytes cultured on target HFWT cells. IL-7 (10 U/mL) was not effective on lymphocyte growth. Without addition of IL-2 to the culture medium, the irradiated HFWT cells themselves could not support survival of the lymphocytes for several days.
We compared the effects of HFWT and MHC class I-non-expressing HCC-2 cells on NK cell expansion using IL-2-containing medium. For 10 d in the culture of PBMC from Subject-1, irradiated HFWT cells induced 72.0% of CD3-CD56+ cells, but irradiated HCC-2 cells could induce only 18.5% of CD3-CD56+ cells. CD16+CD56+ cells shared 72.7% in the former lymphocyte population and, in the latter, 22.8%.
Expansion of human NK cells from PBMCs has long been investigated but large-scale expansions for adoptive immunotherapy of human tumors deserve further investigation. LAK cells have been applied to tumor immunotherapy. However, LAK cell expansion in culture for more than 2 wk usually resulted in the loss of killer cell activity. T cells without killing activity formed a major part of the resulting lymphocyte populations[16]. Similar problems have been found in expansion cultures of tumor-infiltrating lymphocytes, in which cells bearing B cell, NK cell and macrophage markers disappeared early in the culture[17].
The K562 cell line in suspension culture has long been adopted as the common target cell line for determination of the killing activity of human NK cells by the standard 51Cr release assay. For ecological reasons, a nonradioisotopic crystal violet staining assay was used in our experiments for determining the killing activity of CTLs against anchorage-dependent target tumor cells. The crystal violet staining assay is safe and amenable to coculturing effectors and targets for more than 4 h. Since HFWT cells disappeared completely during NK expansion culture described above, crystal violet staining using non-irradiated fresh T cells as the target, was considered as sensitive for assessment of the cytotoxic activity of the NK cells as the standard 51Cr release assay.
The results demonstrated that an anchorage-dependent Wilms tumor cell line is a highly efficient target for selective expansion of human NK cells from PBMCs. After culturing PBMCs from healthy donors for 10-21 d, the number of lymphocytes increased extensively. More than 50% of the resulting population consisted of CD16+CD56+ NK cells that could efficiently kill the MHC class I-non-expressing K562. Moreover, these expanded NK cells also appeared to kill MHC class I-expressing tumor cells (Figure 2, TKB-17 RGB target cells), suggesting the probablity of using these highly active NK cells for adoptive immunotherapy of human tumors.
We have already repeated more than 20 times the 2-wk cultures for NK expansion from PBMCs on HFWT cells. Under microscopic examination, we observed that anchorage-dependent target HFWT cells always disappeared completely, the advantage of which was adoptive immunotherapy with NK cells when compared to the methods of NK cell proliferation with suspension-cultured target tumor cells such as K562 and B lymphoblastoid cell lines.
For NK expansion, direct contact of PBMCs with HFWT cells was required (Table 2), suggesting that factors released from target cells do not contribute to NK cells expansion. The need for direct cell-cell contact had been noted in experiments using K562 cells[18]. Growth stimulation through direct cell-cell contact might not be ascribed simply to molecules on the HFWT cell surface preserved after fixation, although Pierson et al[19] reported that NK cells plated directly on ethanol/acetic acid-fixed M2-10B4, which leaves stromal ligands (cell membrane components and ECM) intact, resulted in increased NK cells expansion compared with medium alone. The variety of the fixation methods used in the present experiments conserved, at least partially, reactivity of the surface molecules. Therefore, some interactions between the surface molecules of HFWT cells may contribute to NK expansion. In any case, contact between PBMCs and live HFWT cells were found to be a key requirement.
NK cells are cytotoxic to tumor and virus-infected cells that have lost surface expression of MHC class I proteins. Target cell expressing MHC class I proteins inhibits NK cytotoxicity through binding to inhibitory NK cell receptors[20,21]. Therefore contrasting properties of NK cell inhibitory receptors compared to CTL T-cell receptors (MHC class I receptors that stimulate, rather than inactivate, the CTL cytotoxic response) are expected to provide complementarity in the cytotoxic response to tumor cells[22,23]. In humans, natural killer (NK) cell function is regulated by a series of receptors and coreceptors with either triggering or inhibitory activity[24].
HFWT cells were more effective targets for NK cell expansion than K562 cells. Since it has been reported that NK cells differentiate from CD34+ progenitor cells[25,26], HFWT cells must stimulate NK cells differentiation from the progenitor cells included in PBMC but not in the CD3-CD56+ subset. Further investigation needs to be conducted to identify the NK precursor cells to which HFWT cells transmit the proliferative signal.
Since the present NK cell expansion culture started as one of variation of the human CTL induction culture from PBMC, the present study has shown that IL-2 is the sole cytokine required for the NK expansion on the T cell layer[27]. In other reports, IL-15 was found to stimulate NK development from CD34+ hematopoietic progenitor cells[28-30]; IL-7 as a cofactor during myelopoiesis, is capable of activating monocytes/macrophages and NK cells[31]. IL-12 showed an inhibitory effect on NK cell growth[32,33]; TGF-β was reported to be similarly inhibitory[34]. The present results suggest that expansion and activation of NK cells may provide an effective immunotherapy of hepatocellular carcinoma.
| 1. | Souza SS, Castro FA, Mendonça HC, Palma PV, Morais FR, Ferriani RA, Voltarelli JC. Influence of menstrual cycle on NK activity. J Reprod Immunol. 2001;50:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Luo DZ, Vermijlen D, Ahishali B, Triantis V, Vanderkerken K, Kuppen PJ, Wisse E. Participation of CD45, NKR-P1A and ANK61 antigen in rat hepatic NK cell (pit cell)mediated target cell cytotoxicity. World J Gastroenterol. 2000;6:546-552. [PubMed] |
| 3. | Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356:1795-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 861] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 4. | Wong KH, Simon JA. In vitro effect of gonadotropin-releasing hormone agonist on natural killer cell cytolysis in women with and without endometriosis. Am J Obstet Gynecol. 2004;190:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Malorni W, Quaranta MG, Straface E, Falzano L, Fabbri A, Viora M, Fiorentini C. The Rac-activating toxin cytotoxic necrotizing factor 1 oversees NK cell-mediated activity by regulating the actin/microtubule interplay. J Immunol. 2003;171:4195-4202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Guven H, Gilljam M, Chambers BJ, Ljunggren HG, Christensson B, Kimby E, Dilber MS. Expansion of natural killer (NK) and natural killer-like T (NKT)-cell populations derived from patients with B-chronic lymphocytic leukemia (B-CLL): a potential source for cellular immunotherapy. Leukemia. 2003;17:1973-1980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Perussia B, Ramoni C, Anegon I, Cuturi MC, Faust J, Trinchieri G. Preferential proliferation of natural killer cells among peripheral blood mononuclear cells cocultured with B lymphoblastoid cell lines. Nat Immun Cell Growth Regul. 1987;6:171-188. [PubMed] |
| 8. | Silva MR, Parreira A, Ascensão JL. Natural killer cell numbers and activity in mobilized peripheral blood stem cell grafts: conditions for in vitro expansion. Exp Hematol. 1995;23:1676-1681. [PubMed] |
| 9. | Porrata LF, Inwards DJ, Lacy MQ, Markovic SN. Immunomodulation of early engrafted natural killer cells with interleukin-2 and interferon-alpha in autologous stem cell transplantation. Bone Marrow Transplant. 2001;28:673-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Sekine T, Shiraiwa H, Yamazaki T, Tobisu K, Kakizoe T. A feasible method for expansion of peripheral blood lymphocytes by culture with immobilized anti-CD3 monoclonal antibody and interleukin-2 for use in adoptive immunotherapy of cancer patients. Biomed Pharmacother. 1993;47:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Malygin AM, Somersalo K, Timonen T. Promotion of natural killer cell growth in vitro by bispecific (anti-CD3 x anti-CD16) antibodies. Immunology. 1994;81:92-95. [PubMed] |
| 12. | Yu Y, Hagihara M, Ando K, Gansuvd B, Matsuzawa H, Tsuchiya T, Ueda Y, Inoue H, Hotta T, Kato S. Enhancement of human cord blood CD34+ cell-derived NK cell cytotoxicity by dendritic cells. J Immunol. 2001;166:1590-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 111] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Kim KY, Kang MA, Nam MJ. Enhancement of natural killer cell-mediated cytotoxicity by coexpression of GM-CSF/B70 in hepatoma. Cancer Lett. 2001;166:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Ucker DS, Obermiller PS, Eckhart W, Apgar JR, Berger NA, Meyers J. Genome digestion is a dispensable consequence of physiological cell death mediated by cytotoxic T lymphocytes. Mol Cell Biol. 1992;12:3060-3069. [PubMed] |
| 15. | Liu SQ, Saijo K, Todoroki T, Ohno T. Induction of human autologous cytotoxic T lymphocytes on formalin-fixed and paraffin-embedded tumour sections. Nat Med. 1995;1:267-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Zhang J, Zhang JK, Zhuo SH, Chen HB. Effect of a cancer vaccine prepared by fusions of hepatocarcinoma cells with dendritic cells. World J Gastroenterol. 2001;7:690-694. [PubMed] |
| 17. | Haas GP, Solomon D, Rosenberg SA. Tumor-infiltrating lymphocytes from nonrenal urological malignancies. Cancer Immunol Immunother. 1990;30:342-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Fink T, Ebbesen P, Koppelhus U, Zachar V. Natural killer cell-mediated basal and interferon-enhanced cytotoxicity against liver cancer cells is significantly impaired under in vivo oxygen conditions. Scand J Immunol. 2003;58:607-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Pierson BA, Gupta K, Hu WS, Miller JS. Human natural killer cell expansion is regulated by thrombospondin-mediated activation of transforming growth factor-beta 1 and independent accessory cell-derived contact and soluble factors. Blood. 1996;87:180-189. [PubMed] |
| 20. | Gumperz JE, Parham P. The enigma of the natural killer cell. Nature. 1995;378:245-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 170] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Katz G, Markel G, Mizrahi S, Arnon TI, Mandelboim O. Recognition of HLA-Cw4 but not HLA-Cw6 by the NK cell receptor killer cell Ig-like receptor two-domain short tail number 4. J Immunol. 2001;166:7260-7267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Long EO, Barber DF, Burshtyn DN, Faure M, Peterson M, Rajagopalan S, Renard V, Sandusky M, Stebbins CC, Wagtmann N. Inhibition of natural killer cell activation signals by killer cell immunoglobulin-like receptors (CD158). Immunol Rev. 2001;181:223-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 113] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Rolstad B, Naper C, Løvik G, Vaage JT, Ryan JC, Bäckman-Petersson E, Kirsch RD, Butcher GW. Rat natural killer cell receptor systems and recognition of MHC class I molecules. Immunol Rev. 2001;181:149-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Bottino C, Falco M, Parolini S, Marcenaro E, Augugliaro R, Sivori S, Landi E, Biassoni R, Notarangelo LD, Moretta L. NTB-A [correction of GNTB-A], a novel SH2D1A-associated surface molecule contributing to the inability of natural killer cells to kill Epstein-Barr virus-infected B cells in X-linked lymphoproliferative disease. J Exp Med. 2001;194:235-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 249] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 25. | Miller JS, McCullar V, Punzel M, Lemischka IR, Moore KA. Single adult human CD34(+)/Lin-/CD38(-) progenitors give rise to natural killer cells, B-lineage cells, dendritic cells, and myeloid cells. Blood. 1999;93:96-106. [PubMed] |
| 26. | Mrózek E, Anderson P, Caligiuri MA. Role of interleukin-15 in the development of human CD56+ natural killer cells from CD34+ hematopoietic progenitor cells. Blood. 1996;87:2632-2640. [PubMed] |
| 27. | Konjević G, Jović V, Jurisić V, Radulović S, Jelić S, Spuzić I. IL-2-mediated augmentation of NK-cell activity and activation antigen expression on NK- and T-cell subsets in patients with metastatic melanoma treated with interferon-alpha and DTIC. Clin Exp Metastasis. 2003;20:647-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Naora H, Gougeon ML. Enhanced survival and potent expansion of the natural killer cell population of HIV-infected individuals by exogenous interleukin-15. Immunol Lett. 1999;68:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Carayol G, Robin C, Bourhis JH, Bennaceur-Griscelli A, Chouaib S, Coulombel L, Caignard A. NK cells differentiated from bone marrow, cord blood and peripheral blood stem cells exhibit similar phenotype and functions. Eur J Immunol. 1998;28:1991-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Lin SJ, Yang MH, Chao HC, Kuo ML, Huang JL. Effect of interleukin-15 and Flt3-ligand on natural killer cell expansion and activation: umbilical cord vs. adult peripheral blood mononuclear cells. Pediatr Allergy Immunol. 2000;11:168-174. [PubMed] |
| 31. | Appasamy PM. Biological and clinical implications of interleukin-7 and lymphopoiesis. Cytokines Cell Mol Ther. 1999;5:25-39. [PubMed] |
| 32. | Liebau C, Merk H, Schmidt S, Roesel C, Karreman C, Prisack JB, Bojar H, Baltzer AW. Interleukin-12 and interleukin-18 change ICAM-I expression, and enhance natural killer cell mediated cytolysis of human osteosarcoma cells. Cytokines Cell Mol Ther. 2002;7:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Satoh T, Saika T, Ebara S, Kusaka N, Timme TL, Yang G, Wang J, Mouraviev V, Cao G, Fattah el MA. Macrophages transduced with an adenoviral vector expressing interleukin 12 suppress tumor growth and metastasis in a preclinical metastatic prostate cancer model. Cancer Res. 2003;63:7853-7860. [PubMed] |
| 34. | Billiau AD, Sefrioui H, Overbergh L, Rutgeerts O, Goebels J, Mathieu C, Waer M. Transforming growth factor-beta inhibits lymphokine activated killer cytotoxicity of bone marrow cells: implications for the graft-versus-leukemia effect in irradiation allogeneic bone marrow chimeras. Transplantation. 2001;71:292-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
Edited by Chen WW Proofread by Zhu LH and Xu FM